An orbital view of covalent bonding
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
Valence bond theory states that
if two atoms have orbitals which contain unpaired electrons then the orbitals will overlap, forming a covalent bond.
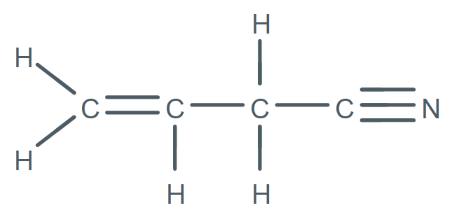
How many sigma and pi bonds does this molecule contain?
9 sigma bonds and 3 pi bonds
When orbitals overlap end-on they form a
sigma bond
When orbitals overlap side-on they form a
pi bond
How many sigma and pi bonds does a double bond contain?
1 sigma and 1 pi
How many sigma and pi bonds does a triple bond contain?
1 sigma and 2 pi
How many sigma and pi bonds does a single bond contain?
1 sigma and 0 pi
classifying sigma and pi bonds is based on rotation:
In a sigma bond, one of the atoms can rotate around the bond axis without affecting the amount of overlap between the two orbitals
In a pi bond, if one of the atoms rotates around the bond axis, the amount of overlap would change
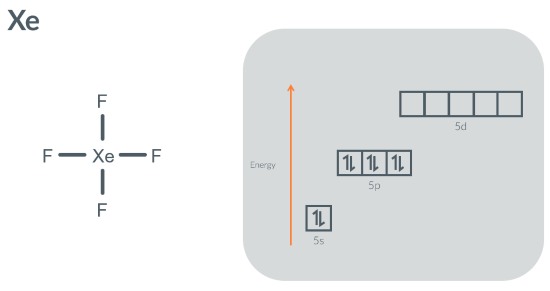
How can Xenon can make 4 bonds in XeF4?
because it can promote 2 electron(s) from a/the 5p orbital(s) to a/the 5d orbital(s)
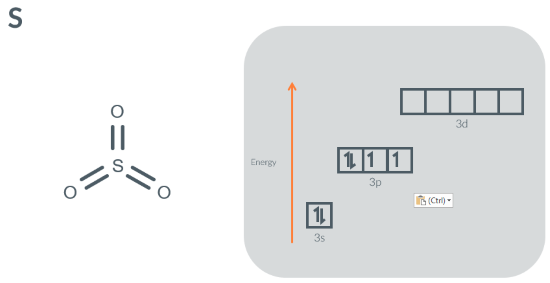
Sulfur can make 6 bonds in SO3 because…
it can promote 1 electron(s) from a/the 3s and 1 electron(s) from a/the 3p orbital(s) to a/the 3d orbital(s)
In Period 3: phosphorus, sulfur and chlorine can all form extra bonds by promoting electrons into their 3d orbitals.
In Period 2: nitrogen, oxygen and fluorine cannot form extra bonds by promoting electrons.
Suggest why.
there are no orbitals close enough in energy to the 2p orbitals
2d orbitals don’t exist