orgo 1 +2 lab final pt.2
1/36
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
37 Terms
Which of the following best describes silica gel stationary phase?
extremely polar
extremely nonpolar
slightly nonpolar
slightly polar
Extremely polar
the distance a solute travels on the stationary phase depends mainly on what molecular characteristics?
Shape
Polarity
Purity
Size
Polarity
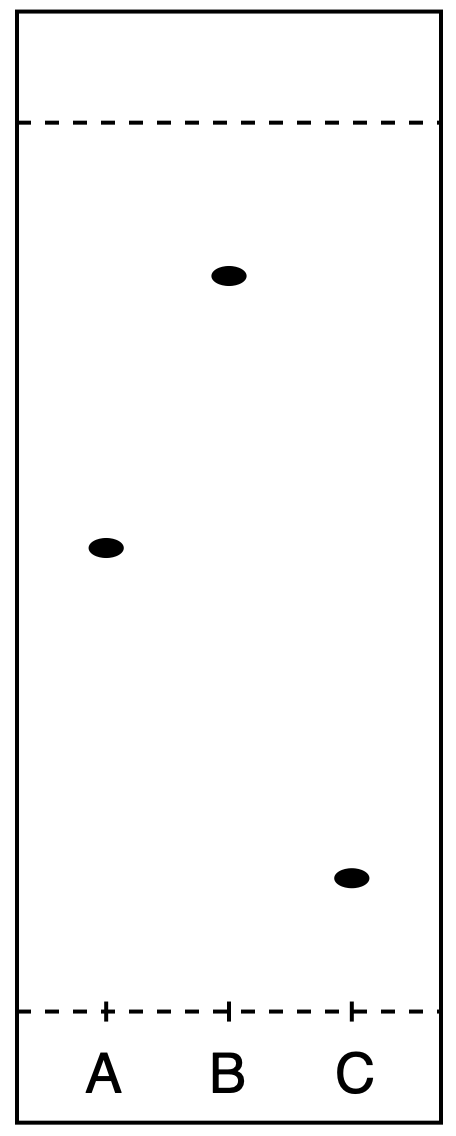
What is the correct ranking of these samples in order of increasing polarity (from least polar to most polar)
C < B < A
B < C < A
C < A < B
B < A < C
B<A<C
T/F: When spotting a sample onto the TLC plate, the size of the spot should be as large as possible
F
Which of the following methods can be used to detect the solute spots on the tlc Plate?
1.Putting the plate under a UV lamp
2. Exposing the plate to a developing stain
3. Feeling the spots on the plate with fingertips
1, 2
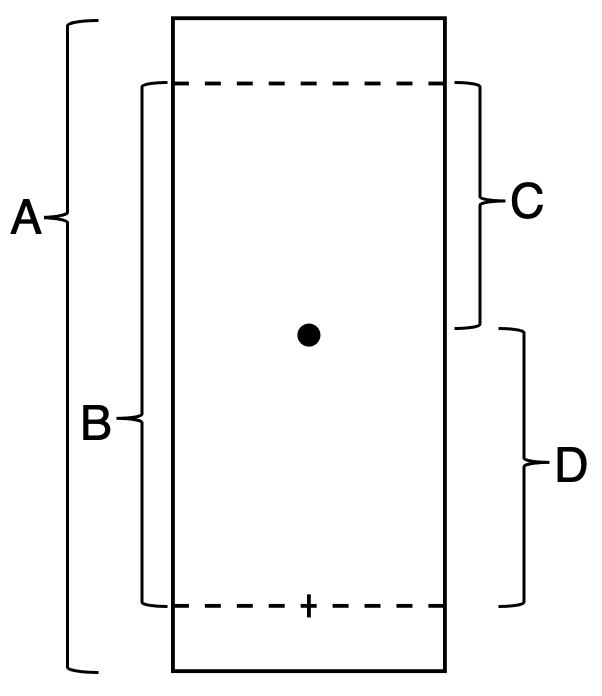
Rf calculation for the spot shown, what is rf =
distance A,B,C,D
divide, minus, plus, multiply
distance A,B,C,D
D/B
Which of the following statements is false?
An extremely polar substance can have an Rf value of 0.
Less-polar substances travel farther than more polar substances.
Some extremely nonpolar substances may have Rf values above 1
The more polar a substance, the lower is its Rf value.
some extremely nonpolar substance may have Rf values above 1
Examine the structures of biphenyl, benzil, and benzoin given in the procedure and pre-lab lecture. Which is most polar?
benzil
biphenyl
benzoin
all have the same polarity
Benzoin
The solvent systems in this experiment will be mixtures of ethyl acetate and hexanes. Which one is Less polar?
ethyl acetate
hexanes
Hexanes

The following silica-gel TLC plate was developed with a 20:80 solvent system (20% ethyl acetate and 80% hexanes). How should the solvent system be changed to allow the components to travel farther up the plate?
make the solvent more polar by increasing the amount of ethyl acetate (30:70)
make the solvent less polar by increasing the amount of hexanes (10:90)
make the solvent more polar by increasing the amount of ethyl acetate (30:70)
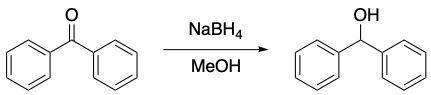
Which statement correctly describes the mechanism of the following reaction?
the nucleophilic carbonyl carbon attacks the electrophilic hydride reagent
the nucleophilic hydride reagent attacks the electrophilic carbonyl carbon
the electrophilic carbonyl carbon attacks the nucleophilic hydride reagent
the electrophilic hydride reagent attacks the nucleophilic carbonyl carbon
the nucleophilic hydride reagent attacks the electrophilic carbonyl carbon
The reaction flask needs to be cooled before adding sodium borohydride because the resulting reaction is highly blank
exothermic
T/F: A properly made ice bath contains both solid ice and liquid water
T
According to the sds, which of the following is/are potential hazards of sodium borohydride?
reacts violently with water
toxic if swallowed
causes severe skin burns and eye damage
reacts violently with water
toxic if swallowed
causes severe skin burns and eye damages
T/F: the sodium borohydride should be weighted at the very beginning of lab and left on the bench top until it is added to the reaction?
F
Considered the structures of the reactant and product. Where should the spots appear on a TLC plate relative to each other?
The reactant and product will have approximately the same Rf values.
The reactant will have a smaller Rf value then that of the product.
The reactant will have a larger Rf value than that of the product.
The reactant will have two spots, while the product will show three spots.
The reactant will have a larger Rf values than that of the product
What solvent system will be used for TLC analysis?
75 % ethyl acetate: 25 % hexanes
10 % ethyl acetate: 90 % hexanes
50 % ethyl acetate: 50 % hexanes
25 % ethyl acetate: 75 % hexanes
25% ethyl acetate, 75% Hexanes
T/F: Once TLC analysis shows the reaction is complete, the solution will be quenched with a mixture of 3M hydrochloric acid and ice
T
How will the diphenylmethanol product be isolated?
TLC
IR spectroscopy
vacuum filtration
simple distillation
vacuum filtration
What change in the IR spectrum would suggest that benzophenone has been converted to diphenylmethanol?
The disappearance of a sharp peak around 1700 cm-1 and the appearance of a broad peak around 3300 cm-1.
The appearance of a sharp peak at 3100 cm-1 only.
The disappearance of a sharp peak around 1700 cm-1 only.
The disappearance of a sharp peak around 3400 cm-1 and the appearance of a broad peak around 1700 cm-1.
the disappearance of a sharp peak around 1700cm and the appearance of a broad peak around 3300cm.
What functional groups react in a fischer esterification reactions?
ester
alcohol
carboxylic acid
acid chloride
alcohol and carboxylic acid
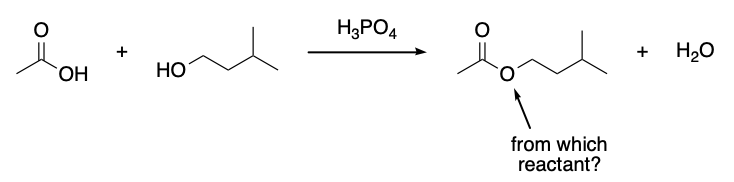
which reactant provides the arrow-indicated oxygen in isopentyl acetate?
acetic acid
phosphoric acid
water
isopentyl alcohol
isopentyl alcohol
Which technique will achieve the long-term heating necessary for this reaction?
simple distillation
vacuum filtration
recrystallization
reflux
reflux
Which of the following remains constant during reflux? Choose apply
moles of reactant
volume of solvent
grams of product
temperature of reaction
volume of solvent and temperature of reaction
What is the procedure for isopentyl acetate reaction?
First, second, third, fourth
secure reaction mixture by clamping round bottom flask neck
collect ir spectra
separate the reaction
heat reaction mixture to refluc
first- secure reaction mixture by clamping round bottom flask neck
second - heat reaction mixture to reflux
third- separate the reaction mixture with DI water
fourth- collect ir spectra
which of the following is the catalyst that will be used in this lab isopentyl acetate experiment?
water
isopentyl alcohol
acetic acid
phosphoric acid
phosphoric acid
Starting with 1.5 mL of isopentyl alcohol, 2.0 mL of concentrated acetic acid, and 0.4 mL of concentrated phosphoric acid, what is the theoretical yield (in g) of isopentyl acetate? Necessary values are given below:
1.794
what is the primary hazard to consider when using concentrated acid
flammable
radioactive
explosive
corrosive
corrosive
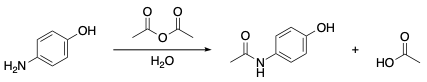
which of the following explains why 4-aminophenol is acetylated at the amine group rather than the phenol group?
the nitrogen is more electronegative than the oxygen
the nitrogen is less sterically hindered than the oxygen
the nitrogen is smaller than the oxygen
the nitrogen is less electronegative than the oxygen
the nitrogen is less electronegative than the oxygen
Starting with 3.00 g of 4-aminophenol (109.13 g/mol) and excess acetic anhydride (102.09 g/mol), what is the theoretical yield (in g) of acetaminophen (151.16 g/mol)?
4.16
which of the following measurement will be used to calculate the percent yield for the reaction acetaminophen?
mass of 4-aminophenol
mass of recrystallized product
mass of crude product
volume of acetic anhydride
mass of 4-aminophenol and mass of recrystallized product
T/F: the purpose of cooling the reaction mixture (steps 5-7) is to avoid heat build-up from the exothermic reaction
F
What solvent system will be used for recrystallizing the crude product?
ethyl acetate and ammonium hydroxide
50% aqueous methanol
100% water
100% acetone
50% aqueous methanol
What is the approximate volume of solvent recommended per 1 g of crude product?
6 mL
4 mL
3 mL
1.5 mL
3ml
What solvent system will be used to develop the TLC plate?
ethyl acetate and ammonium hydroxide
100% acetone
100% water
50% aqueous methanol
ethyl acetate and ammonium hydroxide
According to the SDS, which of the following is/are potential hazards of ammonium hydroxide?
Aquatic toxicity
Serious eye damage
Skin corrosion
Oral and/or respiratory acute toxicity
auqatic toxicity, serous eye damage, skin corrosion, oral and or respiratory acute toxicity
which of the following date will you use to compare the crude product vs the recrystallized product
IR spectra
TLC
Melting point
Recrystallization
ir spectra maybe melting point