4.1 - Atomic Structure and the Periodic Table
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
Define atom
The smallest part of an element that can exist
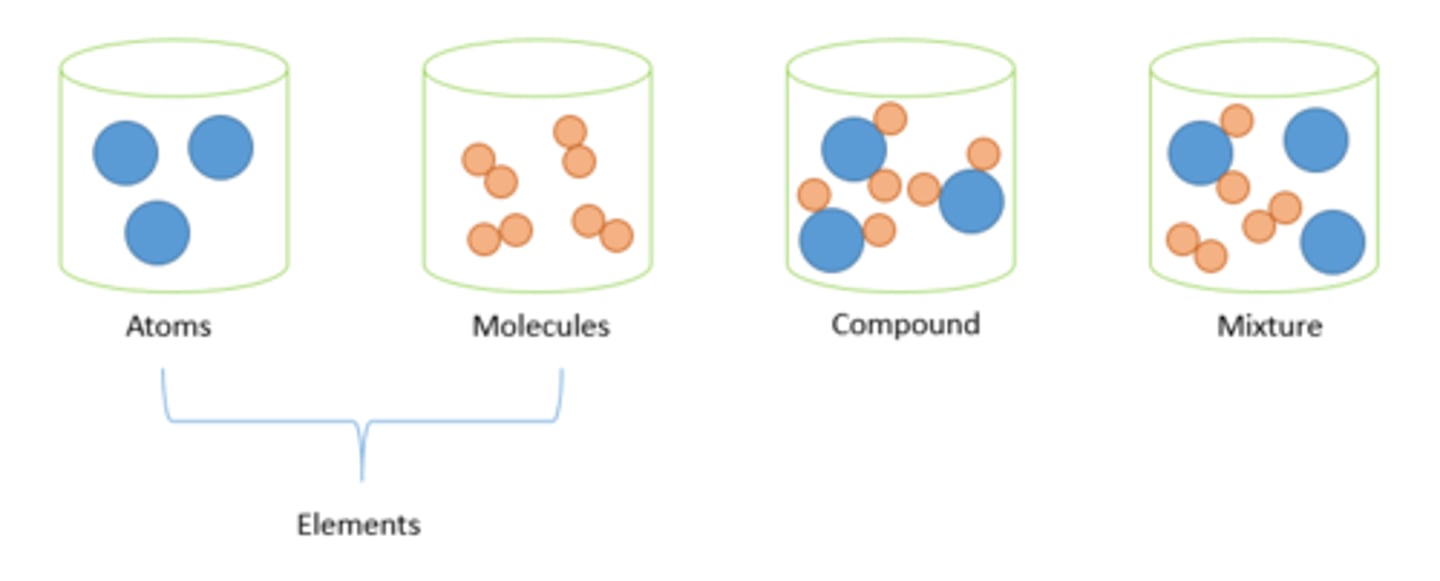
Define element
A substance made up of 1 type of atom
Define compound
Compounds contain two or more elements chemically combined in fixed proportions.
Can be represented by formulae using the symbols of the atoms from which they were formed.
Compounds can only be separated into elements by chemical reactions.
Define mixture
A mixture consists of two or more elements or compounds not chemically combined together.
The chemical properties of each substance in the mixture are unchanged.
Mixtures can be separated by physical processes such as filtration, crystallisation, simple distillation, fractional distillation and chromatography.
These physical processes do not involve chemical reactions and no new substances are made.
Roughly how many elements are there
There are about 100 different elements.
Define isotope
Variations of an element, with the same number of protons and electrons, but different numbers of neutrons
Define molecule
Two or more non-metal atoms, covalently bonded
What is relative atomic mass
The relative atomic mass of an element is an average value that takes account of the abundance of the isotopes of the element.
How do you calculate the Ar (relative atomic mass) of a substance
((Percentage of isotope 1 x mass 1) + (Percentage of isotope 2 x mass 2)…)/100= Ar
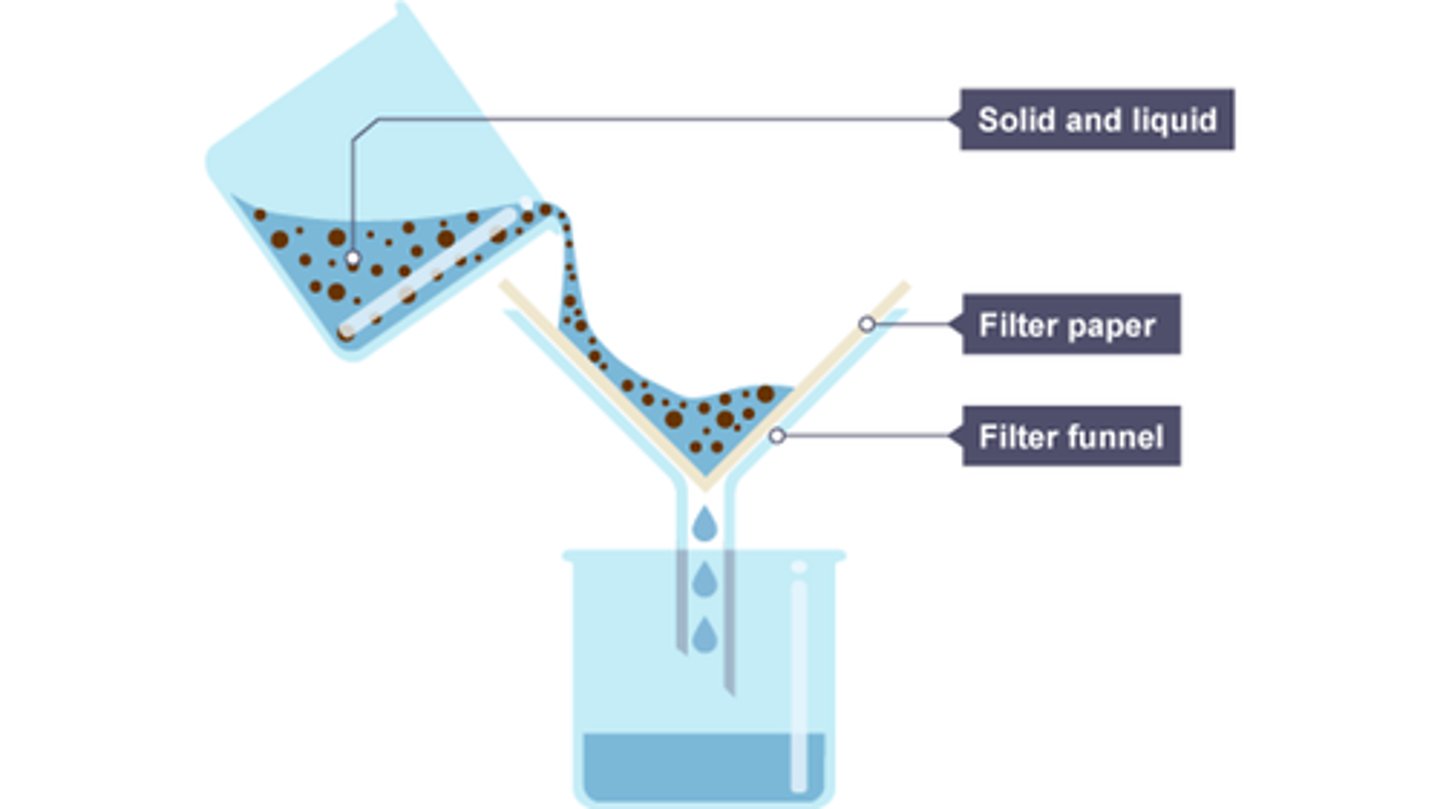
Describe filtration
Filtration is used to separate an insoluble solid is suspended in a liquid. The insoluble solid (called a residue) gets caught in the filter paper, because the particles are too big to fit through the holes in the paper. The filtrate is the substance that comes through the filter paper.
Apparatus: filter paper + funnel.
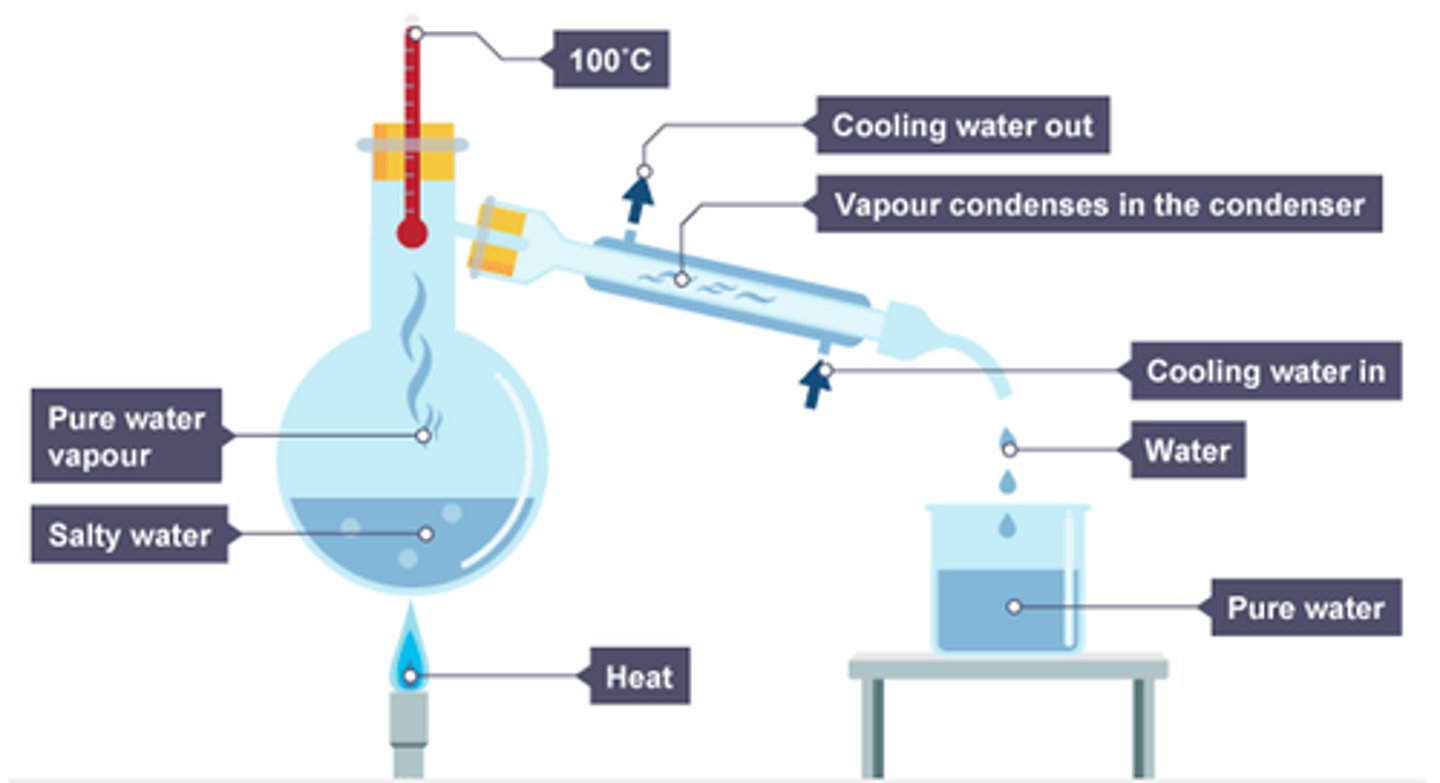
Describe distillation
Simple distillation is used to separate liquid from a solution – the liquid boils off and condenses in the condenser.
The thermometer will read the boiling point of the pure liquid.
Contrary to crystallisation, we get to keep the liquid.
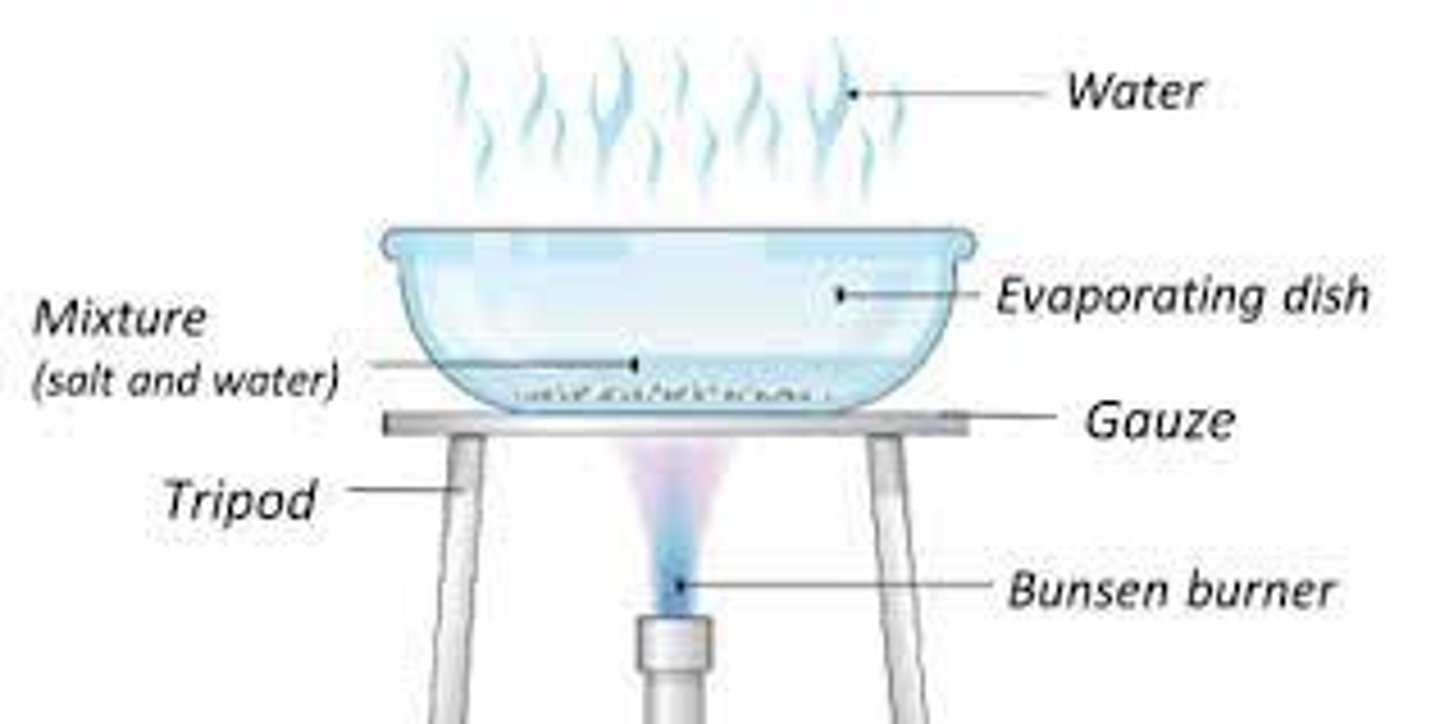
Describe crystallisation
Evaporation is a technique for separation of a solid dissolved in a solvent from a solvent (e.g. salt from H2O).
The solution is heated until all the solvent evaporates; the solids stays in the vessel.
Crystallisation is similar, but we only remove some of the solvent by evaporation to form a saturated solution (the one where no more solid can be dissolved).
Then, we cool down the solution.
As we do it, the solid starts to crystallise, as it becomes less soluble at lower temperatures.
The crystals can be collected and separated from the solvent via filtration.
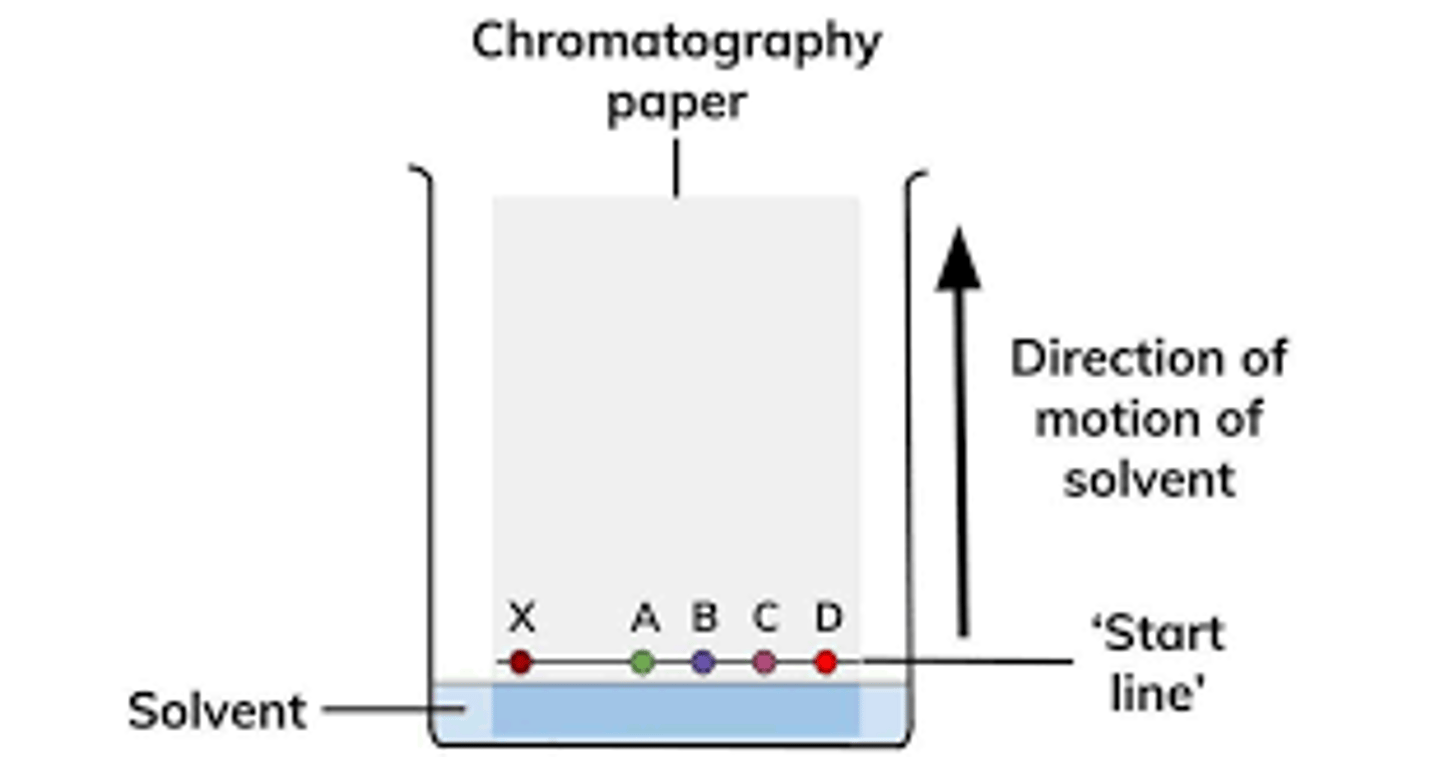
Describe chromatography
Chromatography is used to separate a mixture of substances dissolved in a solvent.
In paper chromatography, we place a piece of paper with a spot containing a mixture in a beaker with some solvent.
The bottom of the paper has to be in contact with the solvent.
The solvent level will slowly start to rise, thus separating the spot (mixture) into few spots (components).
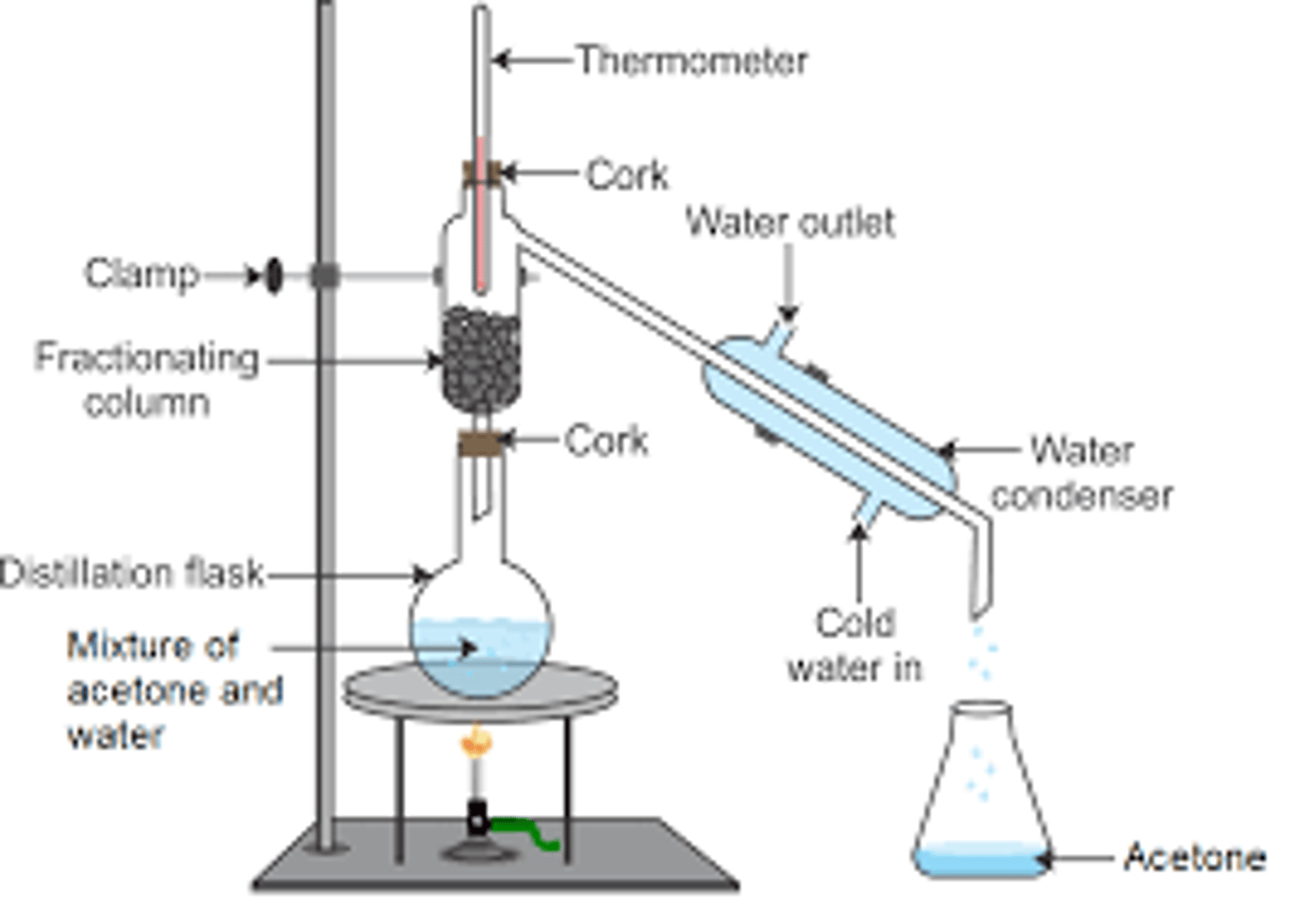
Describe what fractional distillation separates
Fractional distillation is a technique for separation of a mixture of liquids.
It works when liquids have different boiling points.
The apparatus is similar to the one of simple distillation apparatus, with the additional fractionating column placed on top of the heated flask.
The fractionating column contains glass beads. It helps to separate the compounds.
In industry, mixtures are repeatedly condensed and vapourised.
The column is hot at the bottom and cold at the top.
The liquids will condense at different heights of the column
What is a seperating funnel
A separatory funnel is an apparatus for separating immiscible liquids. Two immiscible liquids of different densities will form two distinct layers in the separatory funnel. We can run off the bottom layer (the liquid with greater density) to a separate vessel.
What happens in a chemical reaction
Chemical reactions always involve the formation of one or more new substances, and often involve a detectable energy change
What is the diameter of an atom
1×10-10m
What is the diameter of the nucleus
1×10-14
What are the charges of protons, neutrons, and electrons?
proton-1+
neutron-0
electron-1-
What are the relative masses of protons, neutrons, and electrons?
proton-1
neutron-1
electron-0 (0.0005)
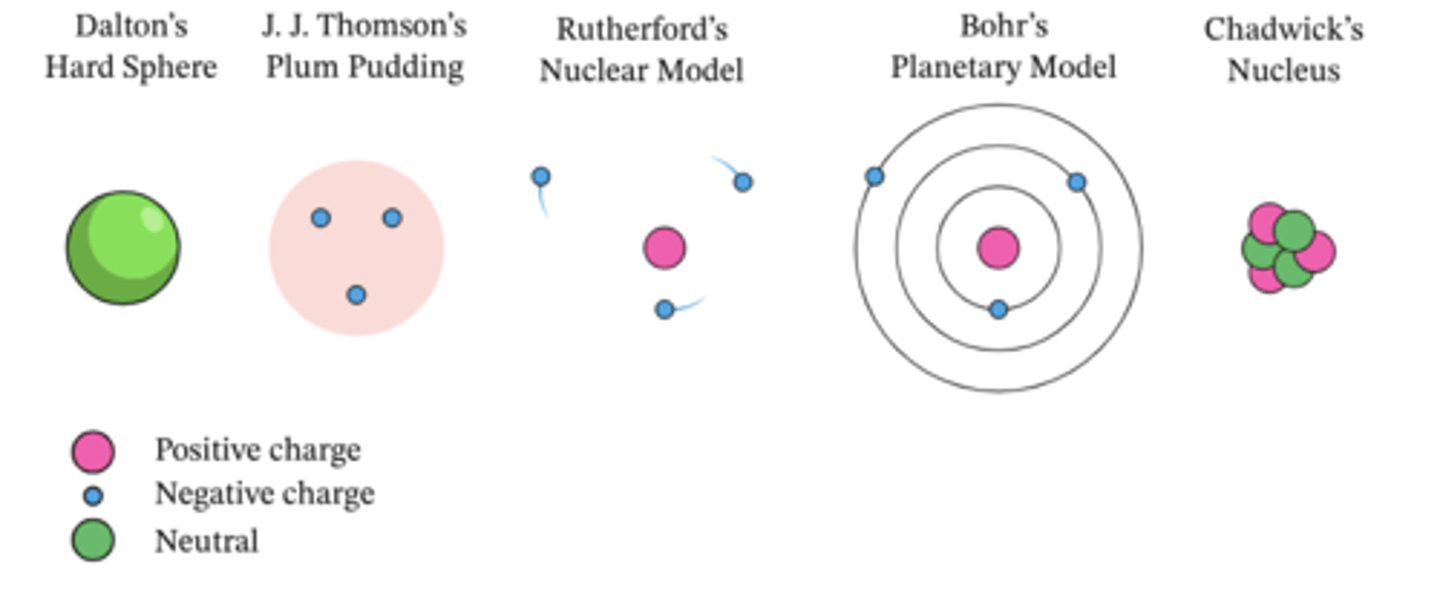
Describe the process of the development of the atom
1803- John Dalton- Hard Ball- Atoms
1903- JJ Thompson- Plum Pudding Model- Electrons
1911- Ernest Rutherford- Nuclear Model (Alpha Particle Scattering experiment)- Positive Nucleus
1913- Niels Bohr- Bohr's Model- Electron Shells
1934- Chadwick- Quantum model- Neutrons
What was the first model of the atom
Before the discovery of the electron, atoms were thought to be tiny spheres that could not be divided.
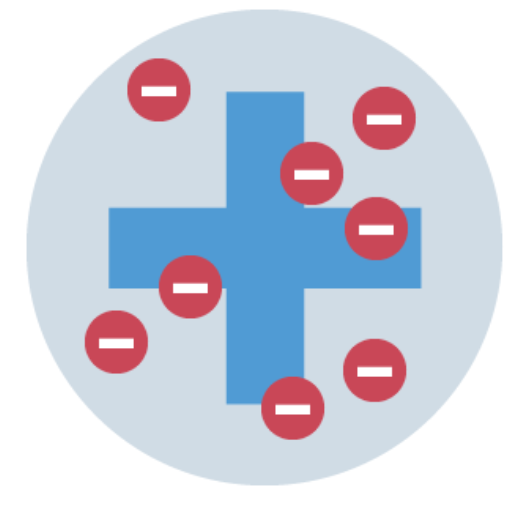
Describe the plum pudding model
The discovery of the electron led to the plum pudding model of the atom.
The plum pudding model suggested that the atom is a ball of positive charge with negative electrons embedded in it.
The mass is evenly distributed
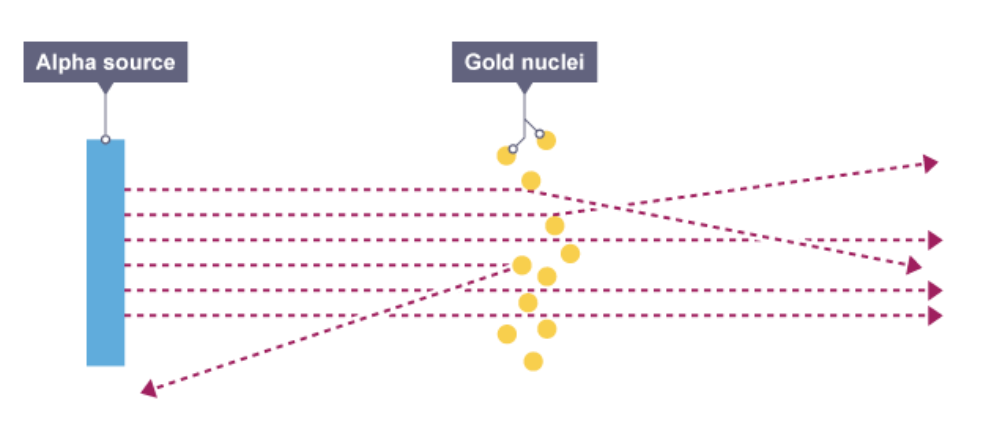
Describe the alpha particle scattering experiment, and what it concluded
Rutherford fired alpha particles, which have a charge of 2+, at a sheet of thin gold foil
Some were reflected, as they hit the gold nucleus, and some were deflected, as they were repelled by the positive charge of the nucleus- this proved the positive charge was concentrated in the nucleus
Most just passed straight through- this proved the atom was mostly empty space
His model, the nuclear model had the mass and positive charge concentrated in the central nucleus, with one layer of electrons orbiting outside- most of the atom was empty space
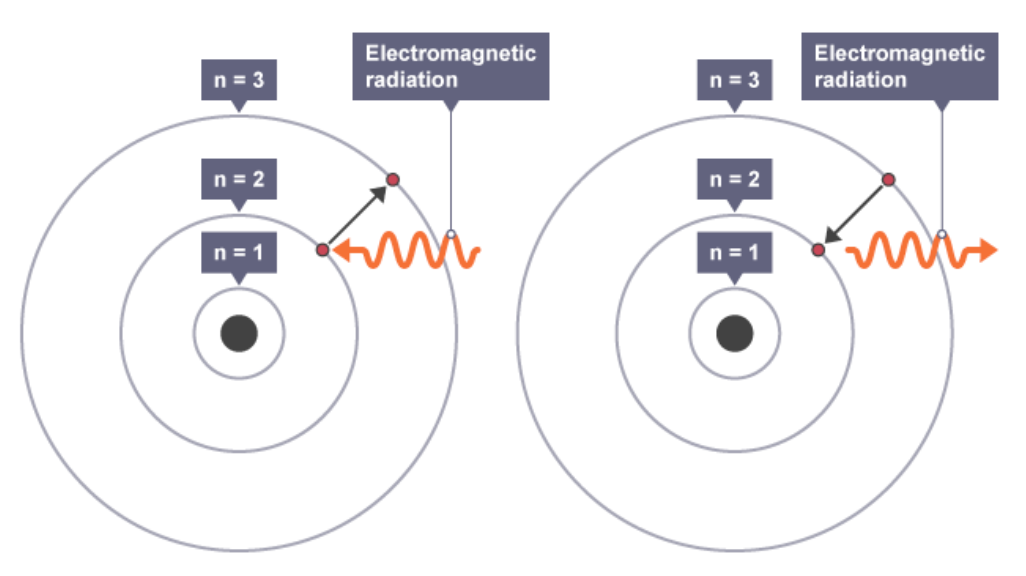
How did Bohr’s model differ from the nuclear model
Niels Bohr adapted the nuclear model by suggesting that electrons orbit the nucleus at specific distances.
The theoretical calculations of Bohr agreed with experimental observations.
What later experiments happened after Bohr
Later experiments led to the idea that the positive charge of any nucleus could be subdivided into a whole number of smaller particles, each particle having the same amount of positive charge.
The name proton was given to these particles.
What did James Chadwick discover
The experimental work of James Chadwick provided the evidence to show the existence of neutrons within the nucleus.
This was about 20 years after the nucleus became an accepted scientific idea.
How did Mendeleev organize his periodic table?
In order of increasing atomic weight
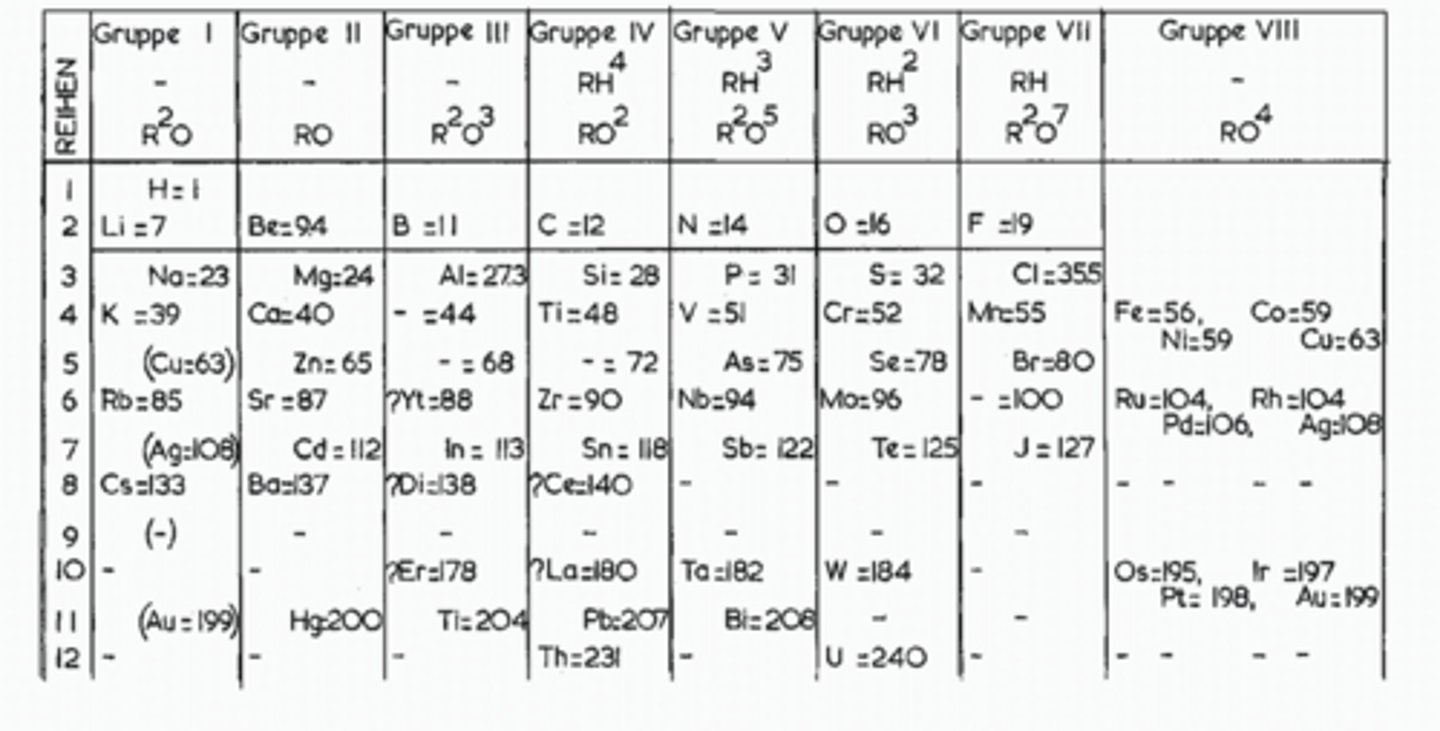
What did Mendeleev do to make the groups' trends work
He swapped elements which didn’t fit the pattern of increasing atomic weight so that groups had similar trends
He left spaces for undiscovered elements, which he could predict the qualities of through its period and group. When these elements, such as gallium, were discovered, and matched his predictions, Mendeleev’s periodic table gained validity.
Which group wasn't on Mendeleev's table and why
Group 0- They are very unnreactive (inert) so were hard to detect, and had not been discovered yet
Why couldn't Mendeleev order his table the way we do
We do it by atomic number (number of protons), but protons hadn't been discovered as it was 1869, so they were still on the hard ball model
Why is the periodic table called the periodic table
The table is called a periodic table because similar properties occur at regular intervals.
What is a metal
Elements that react to form positive ions are metals.
What is a non-metal
Elements that do not form positive ions are non-metals.
Describe the features of group 1
Alkali Metals
Extremely reactive, especially with group 7
Form 1+ ions
Relatively low density (for a metal)
Soft and Dull
Form hydroxides (alkaline solutions) when dissolved in water
1 electron in the outer shell
Reactivity increases down the group
Boiling point decreases down the group
Describe the features of group 7
Halogens
Extremely reactive
Form 1- ions
Form diatomic molecules
Some gases, some liquid, some solid
Often examples in displacement reactions
7 electrons in the outer shell/ 1 less than a full outer shell
Reactivity decreases down the group
Boiling point increases down the group
Describe the features of group 0
The elements in Group 0 of the periodic table are called the noble gases.
They are unreactive and do not easily form molecules because their atoms have stable arrangements of electrons.
The noble gases have eight electrons in their outer shell, except for helium, which has only two electrons.
The boiling points of the noble gases increase with increasing relative atomic mass (going down the group).
Can be used in double glazing (windows), welding, balloons (He), lights and signs (Ne)
Exist in their native state
Explain the trend in reactivity for group 1 and group 7
In group 1, as you move down the group, there are more shells, so there is more electron shielding; this means that the outer electron has less electrostatic attraction between the positive nucleus and the negative outer electron. As a result, it is easier for the atom to transfer its outer electron to another atom and react; this means as you go down group 1, it is easier for the metal to transfer its outer electron, and reactivity increases
As you go down group 7, there are more shells, and therefore more electron shielding; this means there is less electrostatic attraction between the negative outer shell and the positive nucleus. As a result, it is harder for the outer shell to attract the last electron needed, so it is harder for it to react; this means that as you go down group 7, it is harder to attract the last electron to form a full outer shell, so reactivity decreases.
Draw a lithium ion
[2]+
![<p>[2]<sup>+</sup></p>](https://knowt-user-attachments.s3.amazonaws.com/efb939a2-27ec-45ed-a29e-28c1ce951596.png)
Draw a fluoride ion
[2,8]-
![<p>[2,8]<sup>-</sup></p>](https://knowt-user-attachments.s3.amazonaws.com/00913254-4de4-46f7-a055-31c3e7191d37.png)
Describe the physical and chemical properties of transition metals
Chemical Properties
They can form multiple different ions, like Fe2+ and Fe3+
Their ions form different coloured solutions and compounds
They are used as catalysts
Physical Properties
Dense, malleable, ductile
Sonorous
Good conductors of electricity and heat
High melting and boiling points