Histamine and First-Generation Antihistamines
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
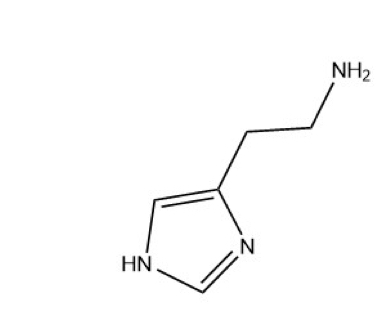
Histamine
Biogenic amine produced from the AA histidine
In invertebrates, it acts as a NT and is involved in vision and sensing
In vertebrates, it plays a role in modulating immunity and gastric function
histamine biosynthesis
Humans lack the enzymes to make histidine denote, so we must get it from our diets
HDC (histidine decarboxylase) converts histidine → histamine
Once produced, histamine is stored at the target tissue and cells
Histamine production can. Be increased. By expression of more HDC enzymes
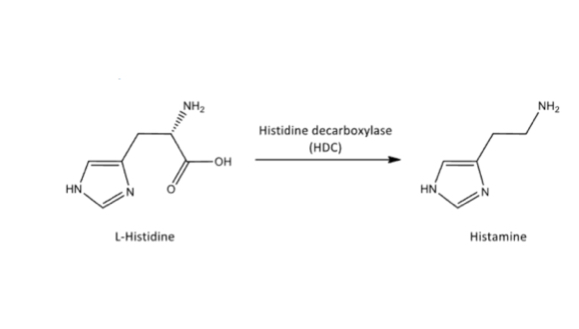
Full mechanism of histamine synthesis
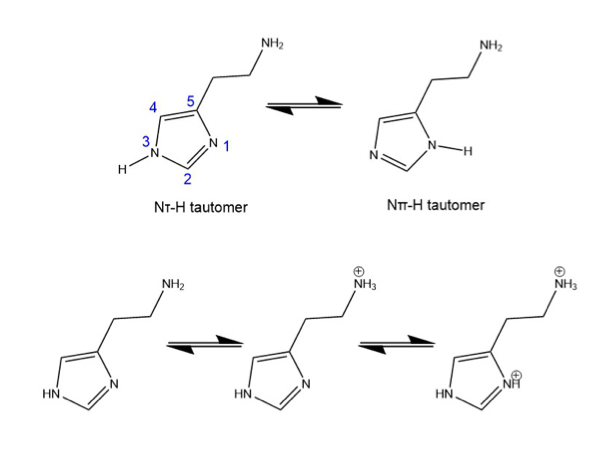
histamine tautomerization
Exists in two tautomeric forms (Nt - protonated at N3. And
Npi - protonated at N1)
Under physiological conditions, the Nt tautomer (monocation) is the predominant form
Tautomerization allows histamine to selectively target different H-receptors
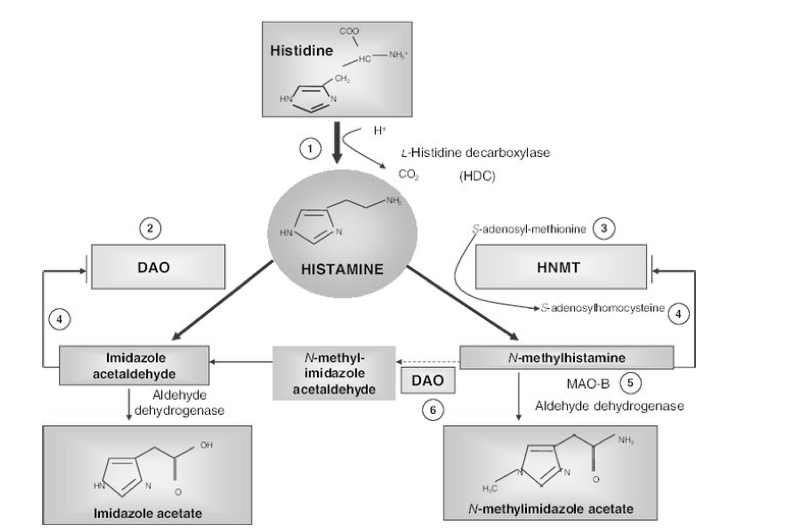
Histamine metabolism
Histamine receptor distribution: H1
Smooth muscle, endothelium, brain
Histamine receptor distribution: H2
Gastric mucosa (gastric parietal cells), cardiac muscle, mast cells, brain
Histamine receptor distribution: H3
Presynaptic autoreceptors and heteroreceptors, brain, myenteric plexus, other neurons
Histamine receptor distribution: H4
Eosinophils, neutrophils, mast cells, CD4 T-cells
Effects of histamine: Lungs
Effect: bronchoconstriction
Clinical manifestation: asthma-like symptoms
Receptor: H1
Effects of histamine: Vascular smooth muscle
Effect: Postcapillary venule dilation, terminal arterials dilation, venoconstriction
Clinical manifestation: erythema
Receptor: H1
Effects of histamine: vascular endothelium
Effect: contraction and separation of endothelial cells
Clinical manifestation: edema, wheal response
Receptor: H1
Effects of histamine: Peripheral nerves
Effect: sensitization of afferent nerve terminals
Clinical manifestation: itch, pain
Receptor: H1
Effects of histamine: heart
Effect: minor increase in contractility and HR
Clinical manifestation: minor
Receptor: H2
Effects of histamine: Stomach
Effect: Increased gastric acid secretion
Clinical manifestation: PUD, heartburn
Receptor: H2
Effects of histamine: CNS
Effect: neurotransmitter
Clinical manifestation: circadian rhythms, wakefulness
Receptor: H3
1st gen antihistamines: basic structure
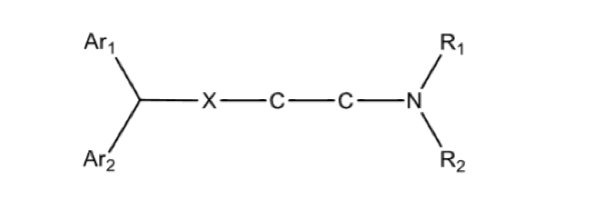
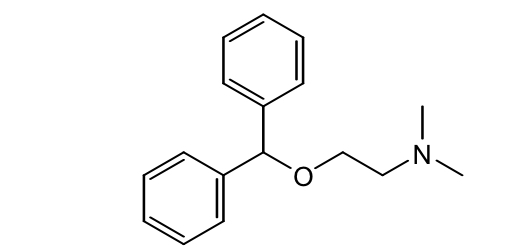
Ethanolamine H1 antihistamines: Diphenhydramine (Benadryl)
Ethanolamine H1 antihistamines: Clementine (Taoist)
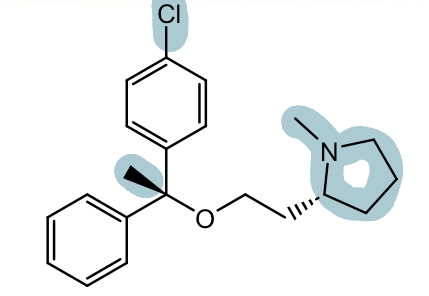
Ethanolamine H1 antihistamines: Doxylamine (unisom, SleepTabs)
Also formulated with pyridoxine (for use in pregnancy) for sleep and morning sickness (Diclegis, Bonjesta)
H1 antihistamines: adverse effects
H1 mediated: decreased NT in the CNS, increased sedation, decreased cognitive and psychomotor performance, increased appetite
Muscarinic mediated: increased dry mouth, urinary retention, and sinus tachycardia (anticholinergic effects)
Alpha adrenergic mediated: hypotension, dizziness, reflex tachycardia
Serotonin receptor mediated: increased appetite
Cardiac-ion channel mediated: prolonged QT intervals, sometimes resulting in ventricular arrhythmias
Preventing drowsiness with H1 antihistamines
To prevent the sedating side effects, diphenhydramine is often formulated as a salt with 8-chlorotheophylline, a mild stimulant
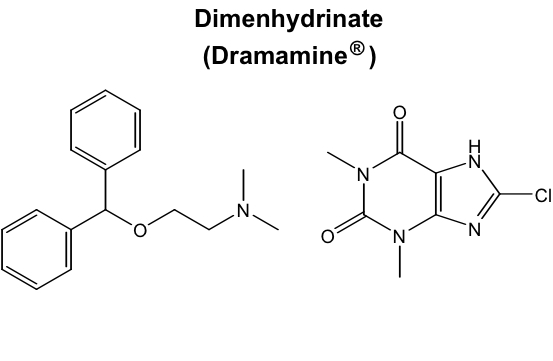
Ethylenediamines: Pyrilamine (mepyramine)
Introduced in the late 1940s for Tx allergy symptoms
Readily crosses BB and causes significant sedation, so it was repurposed as an OTC sleep aid in the 60-70s
Banned as a sleep aid by the FDA in 1989
Still used for Tx cold and menstrual symptoms (Midol complete)
Piperazines: meclizine
Targets H1 receptors at the vestibular nuclei and the vomiting center of the brain in particular, allowing better Tx of vestibular symptoms
Tx and prevention of N/V caused by motion sickness and vertigo
T ½ = 6h (suitable for acute use)
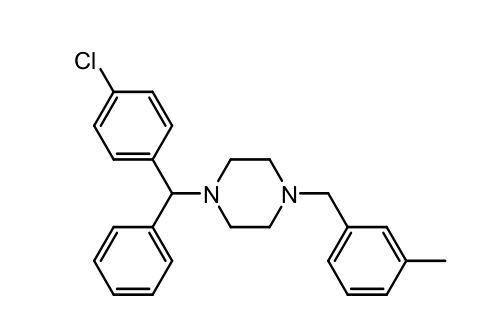
Piperazines: Hydroxyzine
Tx anxiety, pruritis, and is used as a sedative
T ½ = 20h
Different salt formulations are used to change the onset of the drug
Metabolized to cetirizine in the liver
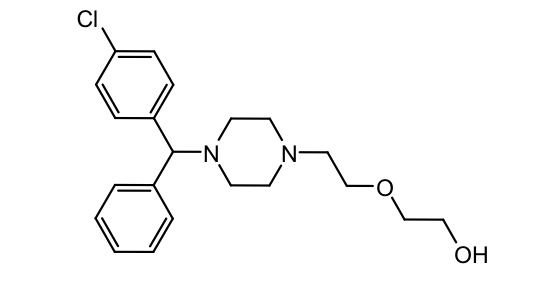
Alkyl amines: Chlorpheniramine
Tx cold, flu, allergy OTC
T ½ = 20h (lower susceptibility to CYP metabolism compared to brompheniramine)
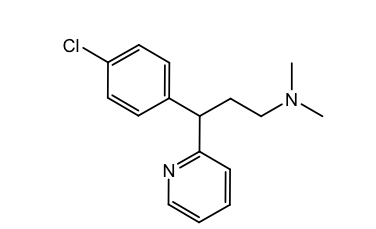
Alkyl amines: brompheniramine
Tx cold, flu, allergies OTC
T ½: 12-16h
Shorter half-life makes it more suitable for pediatric formulations
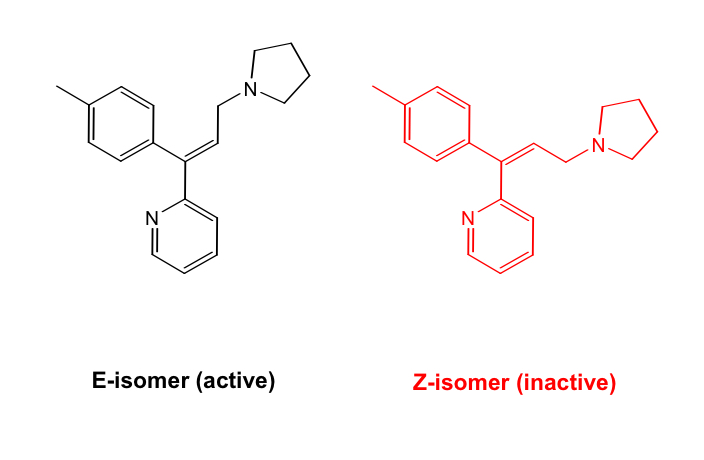
Alkyl amines: Triprolidine
Tx of acute allergic symptoms and common cold
Shorter acting compared to other alkyl amine H1-antihistamines (3-5h) due to reactivity of olefin group
Only one isomer has affirm towards H1 receptor
Tricyclic H1 Antihistamines: promethazine
Phenothiazine derivative
Tx allergic conditions and motion sickness
Strong antiemetic effects due to blockage of dopamine and muscarinic AChR
Readily crosses BBB and has sedative properties
IV admin can cause severe tissue damage and gangrene due to its caustic nature
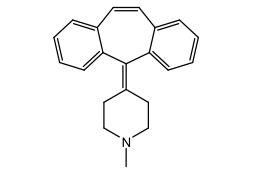
Tricyclic H1 Antihistamines: Cyproheptadine
Piperidine derivative
5-HT2 receptor agonist in addition to H1 receptor activity
Tx allergic rhinitis, appetite stimulant
Tablet or oral solution
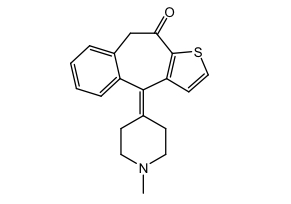
Tricyclic H1 Antihistamines: ketotifen
Piperidine derivative
Mast cell stabilizer in addition to H1 receptor activity
Tx allergic conjunctivitis and asthma prophylaxis
Ophthalmic solution
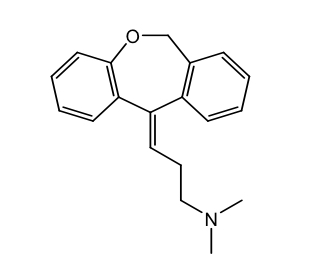
Tricyclic H1 antihistamines: Doxepin
Dibenzoxepin derivative
Oral tablets, topical cream
When taken orally, used as a tricyclic antidepressant, which is also a strong H1 receptor inhibitor
Preferred in cases with insomnia or need for sedation
Used Tx pruritus associated with skin conditions like eczema
Use care in older adults due to risk of oversedation