Signal Transduction I: G-proteins, 2nd messengers, Kinases, Phosphatases
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
Explain stimulus-response at a cellular level
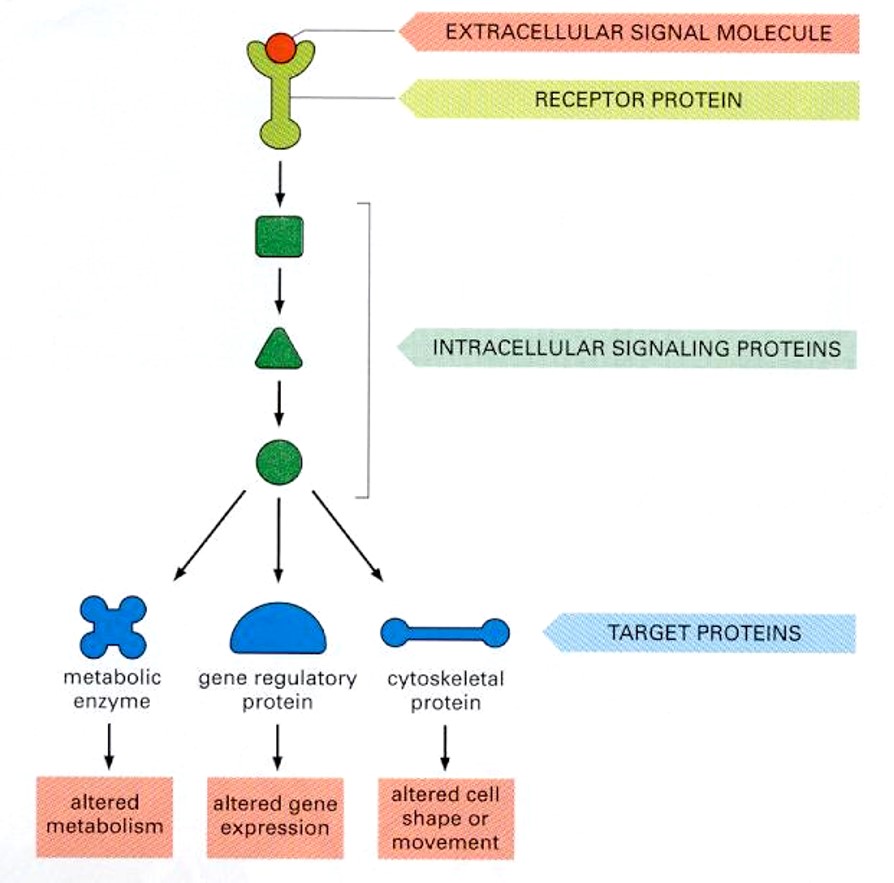
Have a stimulus from an extracellular molecule (ligand) which binds to a receptor protein (GPCR)
Intracellular signaling proteins (transduction components (differ) will target specific proteins (metabolic enzyme, gene regulatory protein etc)
Each target protein will then initiate a response (alter metabolism, alter gene expression etc)
NOTE: Cellular differences of any signal transduction components will alter the response output.
What are the key concepts in signal transduction?
Speed (response time)
Efficiency ([ ], efficacy, amplification)
Specificity (molecular diversity)
2nd messengers and effectors
What are the major intracellular 2nd messenger in signal transduction?
Ca2+
3’,5’-cAMP
3’,5’- cGMP
1,2-Diacylglycerol
Inositol 1,4,5-triphosphate
What % of the genome are GPCR?
>3% (1000-2000 genes)
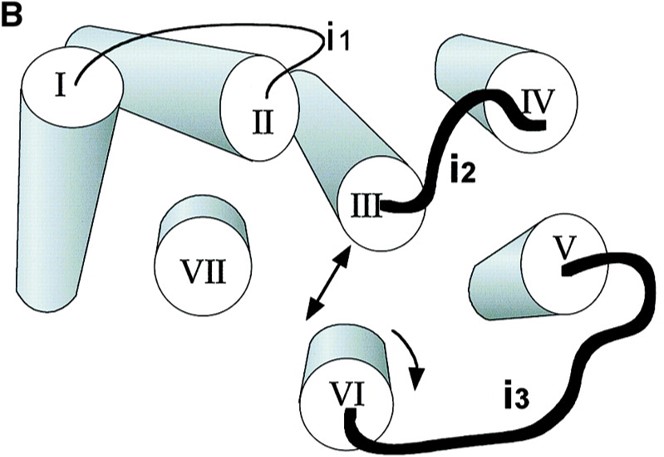
Where does G protein coupling occur?
Intracellular loops (i2 and i3) of the receptor which is exposed during extracellular ligand binding and receptor activation
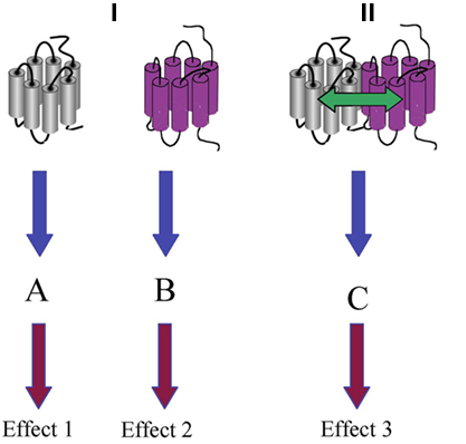
Besides functioning as monomers, how else can GPCRs function?
Homodimers (Same GPCRs)
Heterodimers (Different GPCRs bound together by allosteric interactions)
Oligomers (Same GPCRs)
What structure are GTP-binding proteins (G proteins)?
Heterotrimeric
alpha, beta, and gamma subunits
Lipid anchoring to the lipid bilayer from subunits (prenylation)
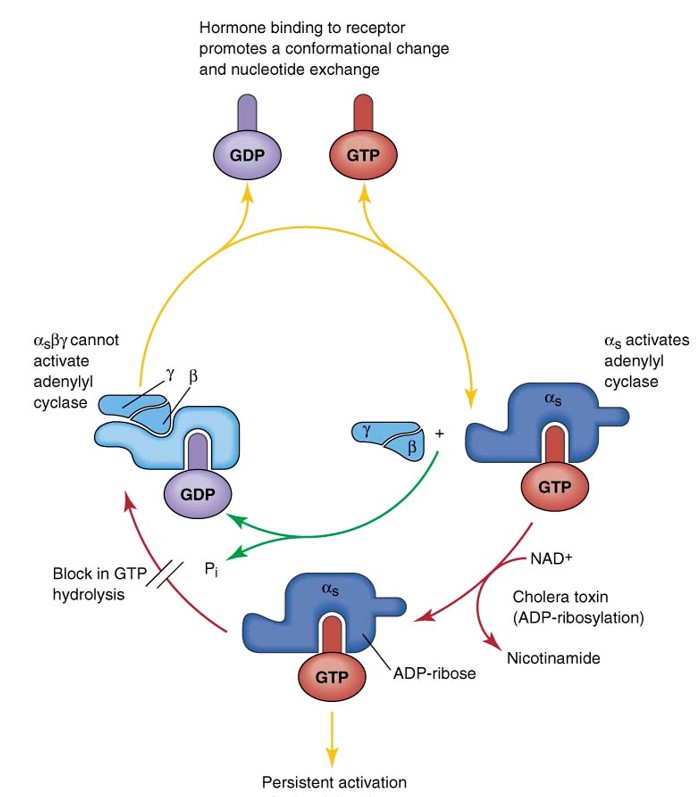
Explain the G-protein cycle: Transduction of 1st messenger signal with GPCRs
Empty GPCR (7 helices with loop structures and heterotrimeric G-protein primed and ready to signal)
Ligand binds (agonist binding), conformational change in loop and helices regions
Facilitates the catalyst exchange of GDP for GTP on α subunit of the heterotrimeric complex.
GTP-bound Gα in the active form and the released GBy dimer can then go on to stimulate downstream effectors.
To restore the system (turn off G-protein), the GTP on the Gα is hydrolyzed to GDP
Original receptor is restored and ready to bind to new ligand
cAMP signaling and Effectors
What turns “on'“ the cAMP signal?
ATP is synthesized by adenylyl cyclase activity (multiple isoforms of these enzymes) & pyrophosphate (release)
Creates a cAMP (monophosphate) molecule which is switched ON
= 3’,5’-cyclic monophosphate
What turns “of” the signal?
cAMP phosphodiesterase & water = Adenosine 5’-monophosphate
Explain the process of Gs couple receptors and production of cAMP
Signal molecule (1st messenger) binds GPCR
Cause conformational change —> trimeric subunits
Gα subunit is bound to GDP which is converted to GTP
Gyb dimer also move but remain up in the bilayer
When Gα bound to the GTP = ACTIVE & binds to adenyl cyclase in the bilayer of the membrane
This activates the GTP-Gα-adenyl-cyclase complex which catalyzes ATP —> cAMP
cAMP & ATP (2nd messengers)
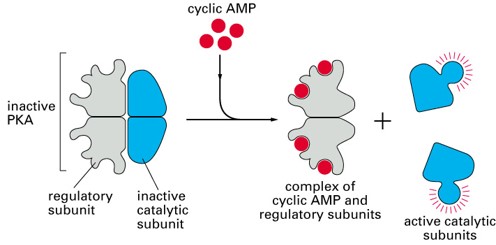
Inactive PKA (consists of 2 regulatory components & two catalytic components) where the catalytic components are activated by ATP & the regulatory components are activated by ATP (2nd messenger effector)
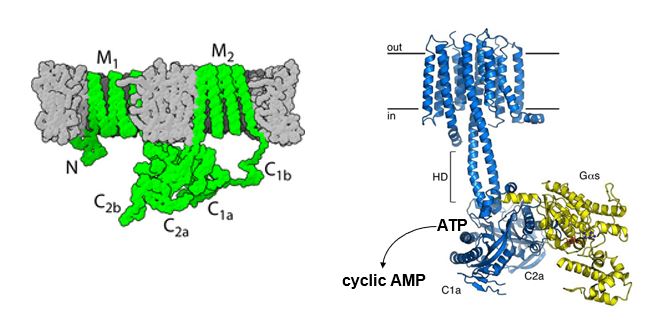
What are the structural features of Adenylyl cyclases?
M1, M2: Transmembrane domains
C: cystolic where G-alpha (yellow) binds to cytosolic domains and allows catalysis of cAMP through ATP modifications (remove 2 PO42- and cyclic monophosphate to produce cAMP)
Name hormone-induced cell responses medicated by cAMP
Thyroid gland: TSH —> thyroid synthesis and secretion
Heart: Adrenaline —> Increase heart rate and force of contraction
Liver: Glucagon —> Glycogen breaks
What are the Effects of Cholera Toxin (CTX) on G protein cycle & cAMP?
CTX contains enzymatic activity that results in covalent modifications of Gs Gα subunit in PTM
Modification is covalent binding of ADP ribose at a specific AA residue (irreversible)
ADP ribosylation of Gs results in loss of GDPase activity (termination) which renders the Gs signaling as ACTIVE indefinitely
Elevates cAMP
NOTE: ADP ribosylation prevents GTP from releasing from Gα. Always active
What happens when Gs function is increased due to Cholera?
Increases adenylyl cyclase (AC) activity
Increases cAMP levels
Increases PKA-mediated phosphorylation
Epithelial Cl- channel (CTFR) is activated by PKA phosphorylation
Increases Cl- secretion into intestinal lumen
Na+ & H20 follow Cl- into lumen and cause diarrhea.
What inhibits adenylyl cyclase?
Gi which inhibits adenylyl cyclase which prevents ATP —> cAMP —> PKA
What is the effect of pertussis toxin (PTX) on G protein cycle?
PTX has an enzymatic activity that results in the covalent modification of Gi/o α subunits.
Modification is also the covalent attachment of ADP ribose at a specific AA (irreversible)
ADP ribsoylation of Gi/Go (Gby and Gα) subunits by PTX block their ability to interact with GPCRs.
PTX blocks Gi/Go coupled signal transduction (can’t inhibit adenyl cyclase & cAMP)
What synthesizes and degrades cAMP?
Synthesis: Adenylate cyclase (AC)
ATP —> cAMP
Degradation: Phosphodiesterase (PDE)
cAMP —> AMP
What is the mechanism of phosphodiesterase?
Hydrolyzes 3’ cyclic phosphate bond of cyclic nucleotide, cAMP, or cGMP. This produces an “inactive” AMP & GMP.
Describe the Phosphodiesterase (PDE) family
conserved catalytic domain
varied specificity towards cAMP & cGMP
Has dual specificity
PDE isoforms are differently expressed and regulated in different tissues
Targets of drug inhibitors (Viagra - prevents cGMP degradation by inhibiting PDE5)
Where do PDE inhibitors bind?
Active site of PDE isoforms
What is the cAMP effector?
Protein kinase A (PKA)
What is the structure of PKA?
2 regulatory subunits that bind to cAMP
2 catalytic kinase subunits that phosphorylate protein substrates (serine, threonine residues)
Alters protein activity/function
Explain the cyclic nucleotide-gated (CNG) ion channels
alters cell membrane potential
major effect in visual & olfactory sensory signaling (cGMP)
Also electric pacemaking activity in hearts cells and neurons (cAMP)
channel activity is increased by direct cyclic nucleotide binding
Some channel isoforms preferably bind cAMP (cardiac isoform), other cGMP (photoreceptor cells, rods, cones)
Primary permeable to Na+ ions
What are the pleiotropic effects of PKA-mediated phosphorylation?
receptors
ion channels
enzymes
TFs
structural proteins
Is PKA mediated phosphorylation reversible?
Yes
cGMP signaling and effectors
What synthesizes and degrades cGMP?
Synthesize: Guanylate cyclase (GC)
Degrades: phosphodiesterase (PDE)
What are the two distinct classes of guanylate cyclase?
Receptor “particulate” (pGC)
prototypical natriuretic peptide (NP) receptor
Soluble (sGC)
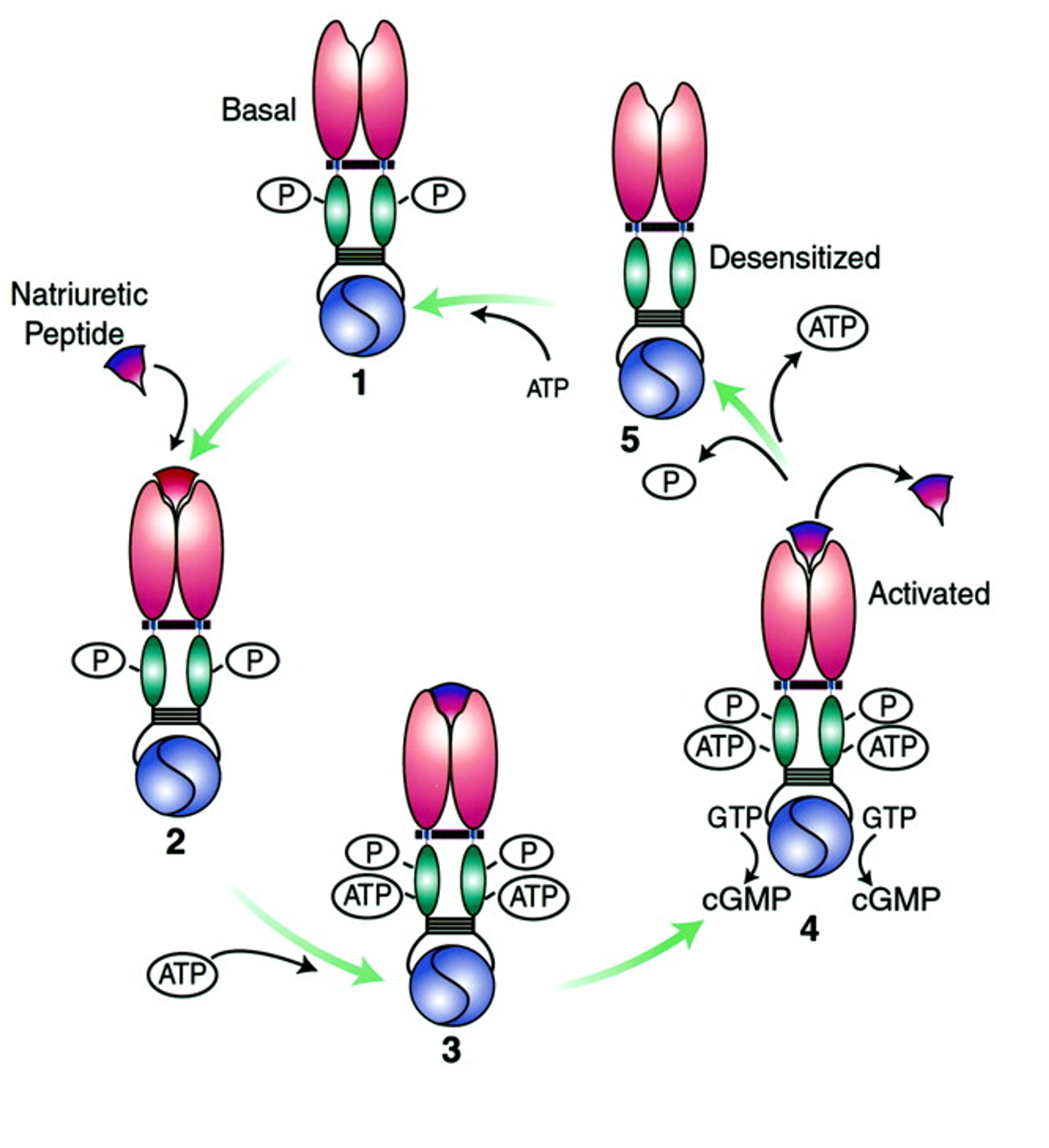
What are the activating steps of natriuretic peptide receptor>
Basal state: Dimerization of the receptor is maintained by phosphorylation of intracellular kinase homology domain (KHD)
Extracellular natriuretic peptide (NP) binds to the receptor dimer
NP binding = conformational change in KHD which allows ATP binding
ATP binding causes conformational changes that induce catalytic activity of guanylate cyclase (GC) domain & production of cGMP
Dissociation of extracellular NP causes dissociation of intracellular ATP & loss of GC activity (ATP and phosphate removal)
What are the activation steps of soluble guanylate cyclase (sGC) causing production of cAMP?
sGC are heterodimers (ab) that possess a heme prosthetic group with a ferrous (Fe2+) core that associates with His105 of the beta-subunit
Nitric oxide (NO) activates sGC by directly binding to ferrous core, breaking the bond between Fe and His —> causes activation of GC domain & production of cAMP.
Termination of sGC activation comes from dissociation of NO from heme prosthetic group
NOTE: CO can also bind to heme group of sGC but less efficacious as an activator of GC domain (doesn’t break Fe-His105 bond)
What is the major cyclic GMP effector?
PKG
Member of serine/threonine protein kinase family
Cytosolic
Phosphorylates multiple proteins at ser/thr residues which alters activation/function
Describe the cyclic nucleotide-gated (CNG) ion channels of cGMP effectors
alters cell membrane potential
Major effector in visual & olfactory sensory signaling
Channel activity is increased by direct cyclic nucleotide binding
primary permeable to Na+ ions
Calcium Signaling & effectors
Explain stimulation of phospholipidase (check this!)
Signal molecule (ligand) binds to GPCR on CM causing a conformational change in the receptor.
This activates the receptor and allows it to interact with nearby Gq protein
Activated GPCR promotes exchange of GDP —> GTP on α subunit of Gq protein = activation.
GTP-bound α subunit (Gqα) dissociates from the βγ subunits and interacts with downstream effectors.
Activation of Gqα activates the enzyme phospholipidase which is membrane-bound.
Phosphatidylinositol 4,5-biphosphate (PI(4,5)P2) is hydrolyzed by the catalysis of phospholipidase
inositol: diffuses through cytosol
DAG: Lipid remains in the membrane and activates other signaling proteins
Inositol binds to a calcium-gated channel on the membrane of ER which opens the channels and allows Ca2+ to be released into cytosol (increases intracellular [ca2+]).
DAG in the plasma membrane elevates Ca2+ levels by activating PKC (phosphorylates target proteins - gene expression, metabolism, secretion)
What are protein Kinase C (PKC)?
They are ser/thr kinases with multiple substrates
How can you measure intracellular calcium?
Flourescent calcium-sensitive indicator dye (Fura-2)
What is the sources Ca2+ levels in cell signaling?
Ca2+ sources for cell signaling
Extracellular Ca entry
large electrochemical driving force for ca entry through ion channels.
Release of Ca from intracellular storage organelles (ER, SR)
What are the maintenance mechanisms of Ca2+?
Plasma membrane caATPase pumps & exchangers that extrude ca@+ at metabolic cost (ATP)
Organelle CaATPase pumps that requester Ca2+ also at a metabolic cost (ATP)
INtracellular Ca2+ binding proteins (CaBP’s)
What do elevated Ca2+ levels bind to?
Calmodulin
What does binding of Calmodulin do?
Activates Ca-CaM dependent protein kinase
Explain the Ca-CaM dependent kinase pathway steps
Calmodulin molecule where Ca2+ binds to form Ca2+/calmodulin complex
Binds to a ca-CaM dependent protein kinase (activated)
The protein undergoes auto-phosphorylation (ATP —> ADP) where the protein is now FULLY ACTIVE
Then Ca2+ is released, cal is released, and the protein remains active because of the auto-phosphorylation
then protein undergoes dephosphorylation through phosphatase = inactive
Explain Desensitization of GPCR’s via GRK-mediated phosphorylation and Arrestin binding
Phototransduction
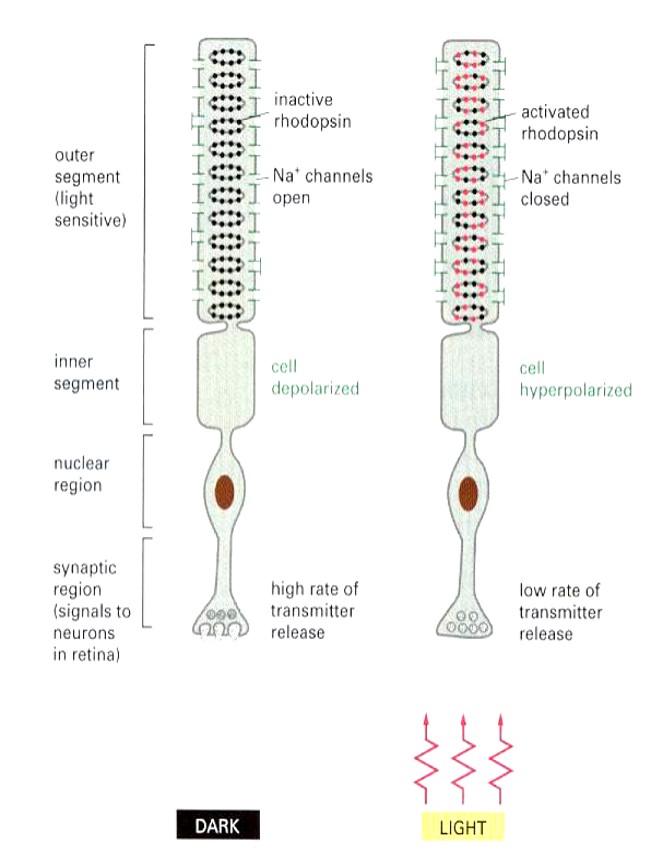
Explain Rhodopsin
Located in rods and cons of eye
light-activated GPCR
Via photo-sensitive covalently attached ‘ligand’ (11 cis chromophore —> 11 trans chromophore)
attached to Lys 296
Explain Rhodopsin activation
When light hits 11 chromophore, it undergoes conformational change to trans which triggers activation of rhodopsin (one rhodopsin absorbs one photon)
Activated rhodopsin activates 500 transducin (G-protein) which facilitates GDP —> GTP
α-subunit (Gα) dissociates from the βγ subunits of transducin.
α-subunit (Gα) bound GTP activates 500 PDE (G-protein effector)
cGMP is hydrolyzed (2nd messenger)
250 Na+ channels close (2nd messenger effector - CNG ion channel)
10^6 Na+ ions/s are prevented from entering cell (about 1s long)
Rod cell membrane is hyperpolarized by 1mV