Memorization for Final Exam
1/27
Earn XP
Description and Tags
CHEM:1070
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
strong acids
HCl
HBr
HI
HClO4
HNO3
H2SO4 -
weak acids
HF
HCN
H2CO3
H3PO4
HNO2
hydroxide
OH-
ammonium
NH4+
nitrate
NO3-
nitrite
NO2-
perchlorate
ClO -
chlorate
ClO3 -
chlorite
ClO3 -
hypochlorite
ClO4 -
carbonate
CO3 2-
bicarbonate
HCO3 -
cyanide
CN -
acetate
C2H3O2 -
sulfate
SO4 2-
bisulfate
HSO4 -
sulfite
SO3 2-
bisulfite
HSO3 -
phosphate
PO4 3-
biphosphate
HPO4 2-
dihydrogen phosphate
H2PO4 -
phosphite
PO3 3-
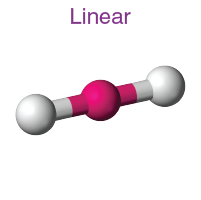
linear - linear
electron groups - 2
bonded atoms: 2
lone pairs: 0
angle: 180
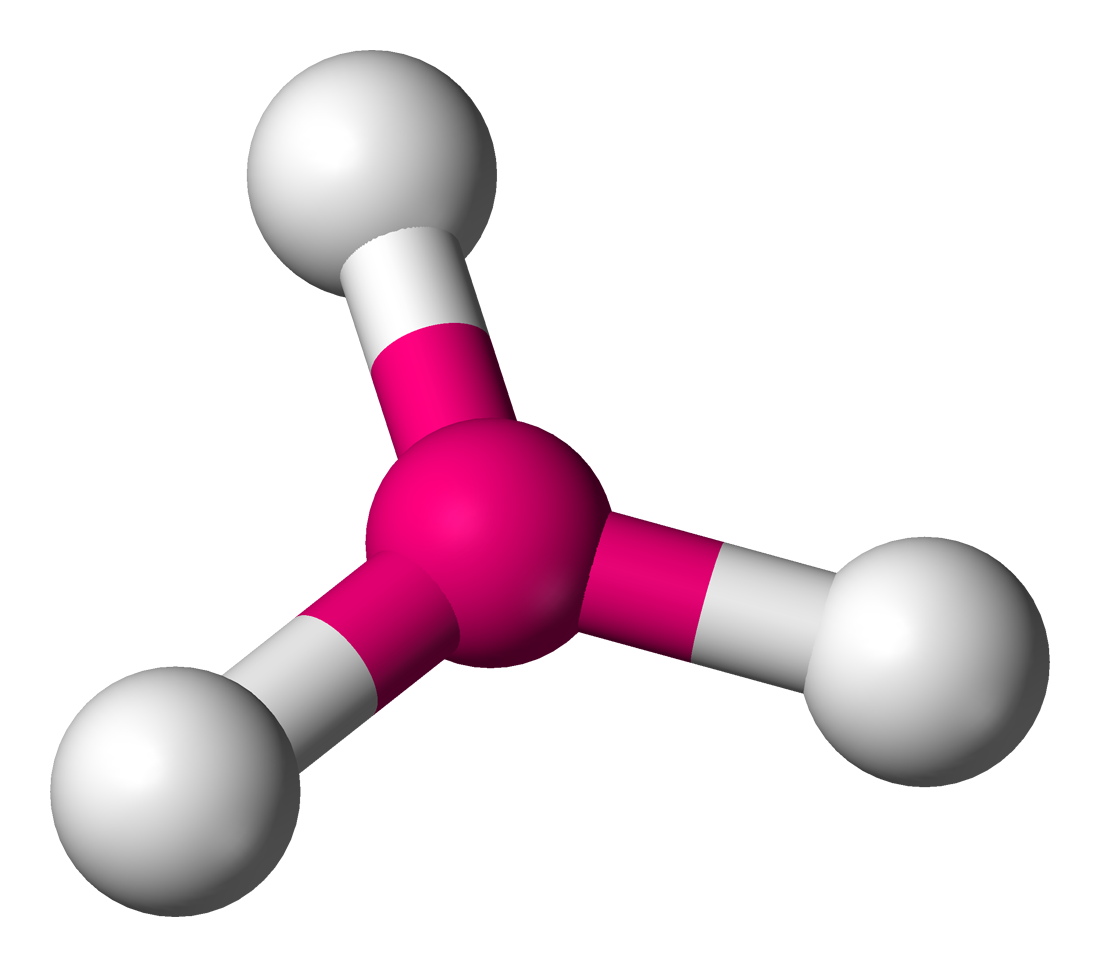
trigonal planar - trigonal planar
electron groups: 3
bonded atoms: 3
lone pairs: 0
angle: 120
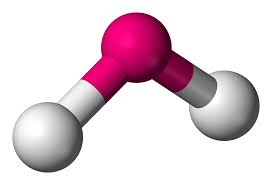
trigonal planar - bent
electron groups: 3
bonded atoms: 2
lone pairs: 1
angle: 120
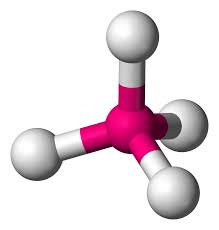
tetrahedral - tetrahedral
electron groups: 4
bonded atoms: 4
lone pairs: 0
angle: 109
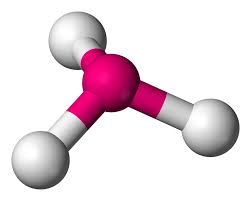
tetrahedral - trigonal pyramidal
electron groups: 4
bonded atoms: 3
lone pairs: 1
angle: 109
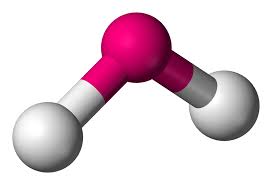
tetrahedral - bent
electron groups: 4
bonded atoms: 2
lone pairs: 2
angle: 109