Required practicals
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
The first step in preparing a soluble salt is to add powdered insoluble reactant to ___ in a beaker until it is in ___
Acid, excess
The second step in preparing a soluble salt is to ___ the mixture in the beaker into a ___ ___ and collect the ____
Filter, conical flask, filtrate
The third step in preparing a soluble salt is to pour the filtrate into an ___ ___ which is then placed on a water bath and heated over a ___ ___ until half the water has ___
Evaporating basin, Bunsen burner, evaporated
The fourth step in preparing a soluble salt is to pour the remaining solution into a ___ ___ and leave it in a warm dry place for ___ to occur
Watch glass, crystallisation
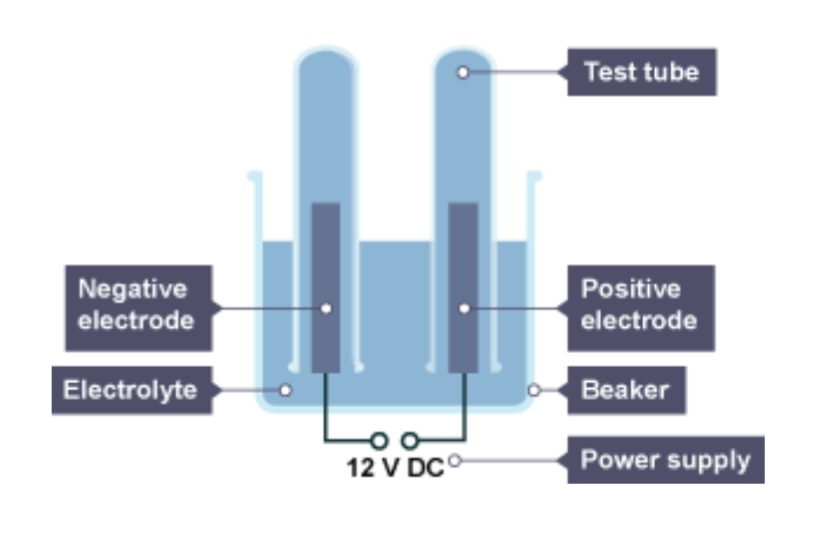
What will be produced at the cathode of the metal is less reactive than hydrogen?
The metal
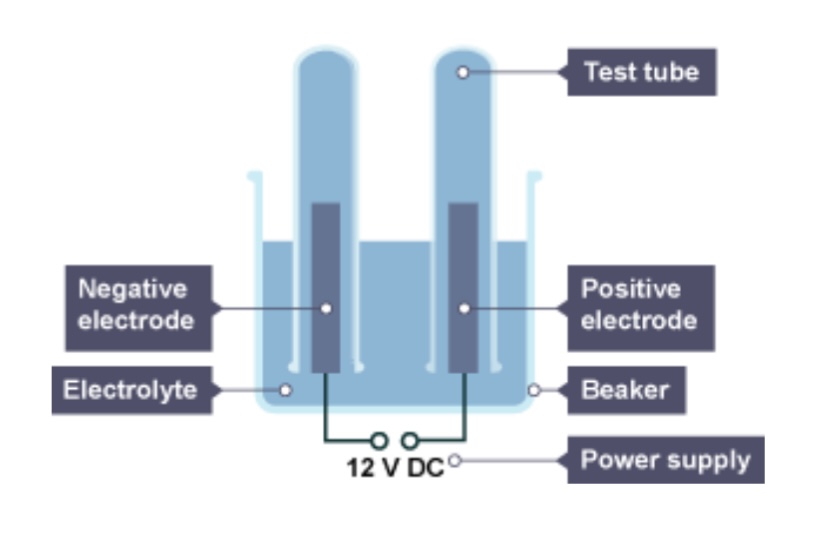
What will be produced at the cathode if the metal is more reactive than hydrogen?
Hydrogen
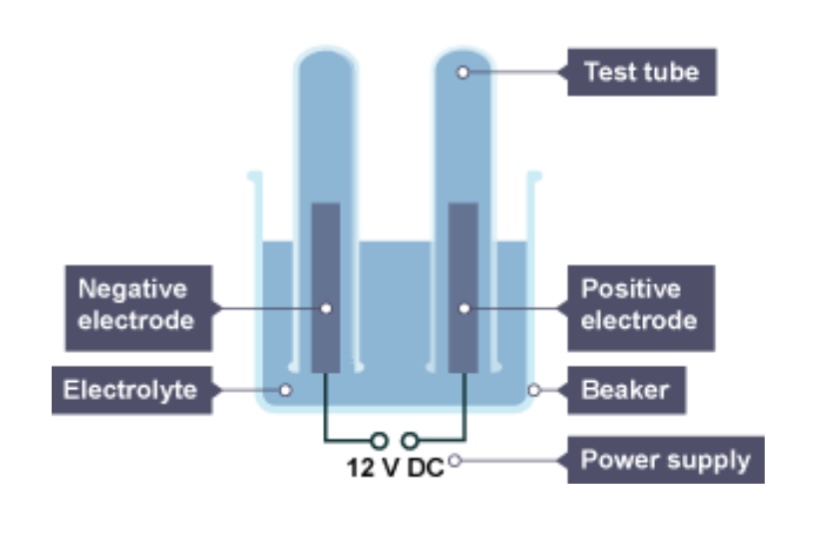
If there is a group 7 element, what will be released as a gas at the anode?
The group 7 element
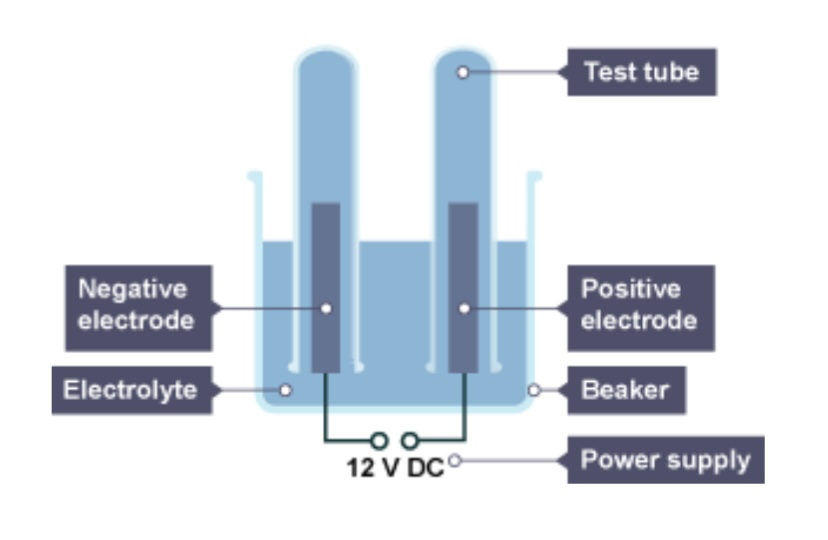
If there isn’t a group 7 ion, which gas will be released at the anode?
Oxygen
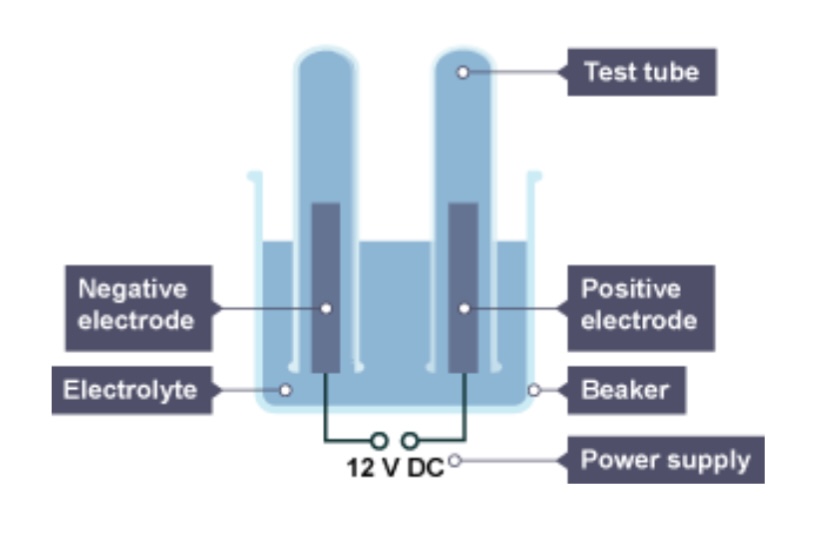
The positive electrode is attached to the ___ terminal of the dc power pack
Positive
A ____ is used to determine the volumes of acid and alkali that must be mixed in order to obtain a solution containing only salt and water
Titration
The first step of titrations is to use a pipette and pipette filler to add ___ ___ solution to a clean ___ ___
Sodium hydroxide, conical flask
The second step in titrations is to add a few drops of ____ and put the conical flask on a ___ ___
Indicator, white tile
The third step of titrations is to fill the ____ with hydrochloric acid and note the ___ ___
Burette, starting volume
The fourth step of titrations is to slowly add the acid from the burette to the alkali in the conical flask ……
Swirling to mix
The fifth step of titrations is to stop adding the acid when the __ __ is reached, noting the final __
End point, volume
What is it called when the indicator first permanently changes colour during titrations?
The end point