Chem 245 Flashcards
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
64 Terms
Qualitative / Quantitative Elemental Analysis
Qualitative EA: what type of atoms are present?
Quantitative EA: how much of each type of atom is/are present?
The combination of both provides the empirical formula
Empirical vs Molecular Formula
Empirical Formula: represents the simplest whole number ratios of elements present in a molecule
Molecular Formula: Chemical formula representing the exact number and types of each element in a single compound
both don’t provide any structural or connectivity analysis
Calculating Empirical Formula
Write out balanced combustion equation
CxHyO2 + H2O (excess) —> xCO2 + y/2•H2O
Calculate the mmol of the atomic elements present in the unknown sample
1:1 ratio for CO2 and C, and 1:2 ratio for H2O and H
Calculate the mass (in mg) of the atomic elements present in the unknown sample
Calculate the weight% of the atomic elements present in the unknown sample
Check to see if the weight % sums to 100%. If they don’t, the difference is the weight % of O
Assume you have 100g of material. The % now equals grams of each element. Calculate the moles of each element
Calculate the empirical formula by dividing through by the lowest mole value. Round to the nearest whole number
Nitrogen Rule
If the amu value (or M.W) is an odd number, you either have
one nitrogen
an odd number of nitrogen atoms (1,3,5,7…)
If the amu value (or M.W) is an even, you either have
0 nitrogen atoms
an even number of nitrogen atoms (0,2,4,6…)
Index of Hydrogen Deficiency (IHD)
look at how many H atoms are expected, then look at how many are actually present
In saturated alkanes, the molecular formula should be CnH2n+2
A unit of unsaturation is added for each multiple bond in a molecule or ring
IHD of 4 or more, think aromatic ring
Heteroatom
Anything that’s not carbon or hydrogen
Index of Hydrogen Deficiency (IHD): What to add/remove for each heteroatom
If element included is in:
group 5 (N-Bi), add one H to the molecular formula
group 6 (O-Te), no change is required
group 7 (F-I), subtract one H
Rule of 13
Mass spec (HRMS) provides the exact mass (in amu)
We translate n and r into CnHn+r and
U= (n-r+2)/2
U= units of unsaturation
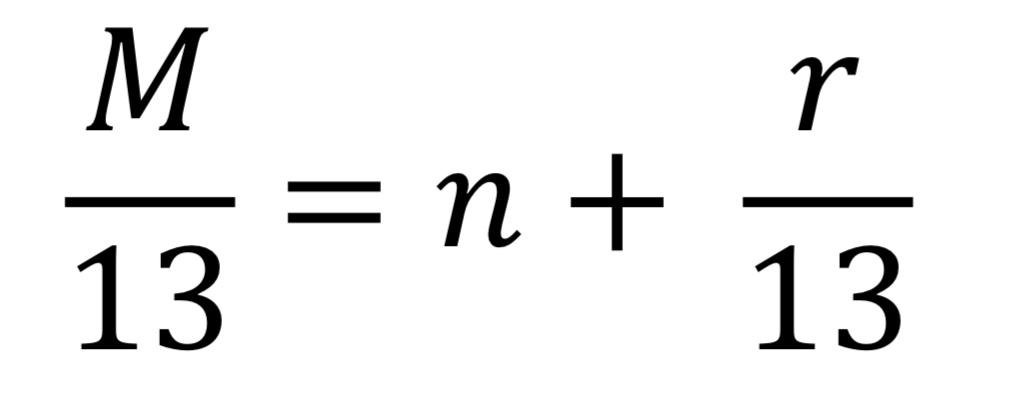
Gas Chromatography Mass Spectrometry (GC-MS)
compounds vaporized into gas, carried through long columns using inert He gas (mobile phase)
inert: doesn’t react
compounds interact differently with the column, resulting compounds eluting at various times (retention times)
once these compounds elute, they undergo analysis using MS
Key information:
Product formation: a new spot formed
Reaction completion: loss of all starting material
Side reactions: New spot thats not product or starting material
reaction monitoring: how long does a reaction take? How may equivalents are required?
General Schematic of an MS
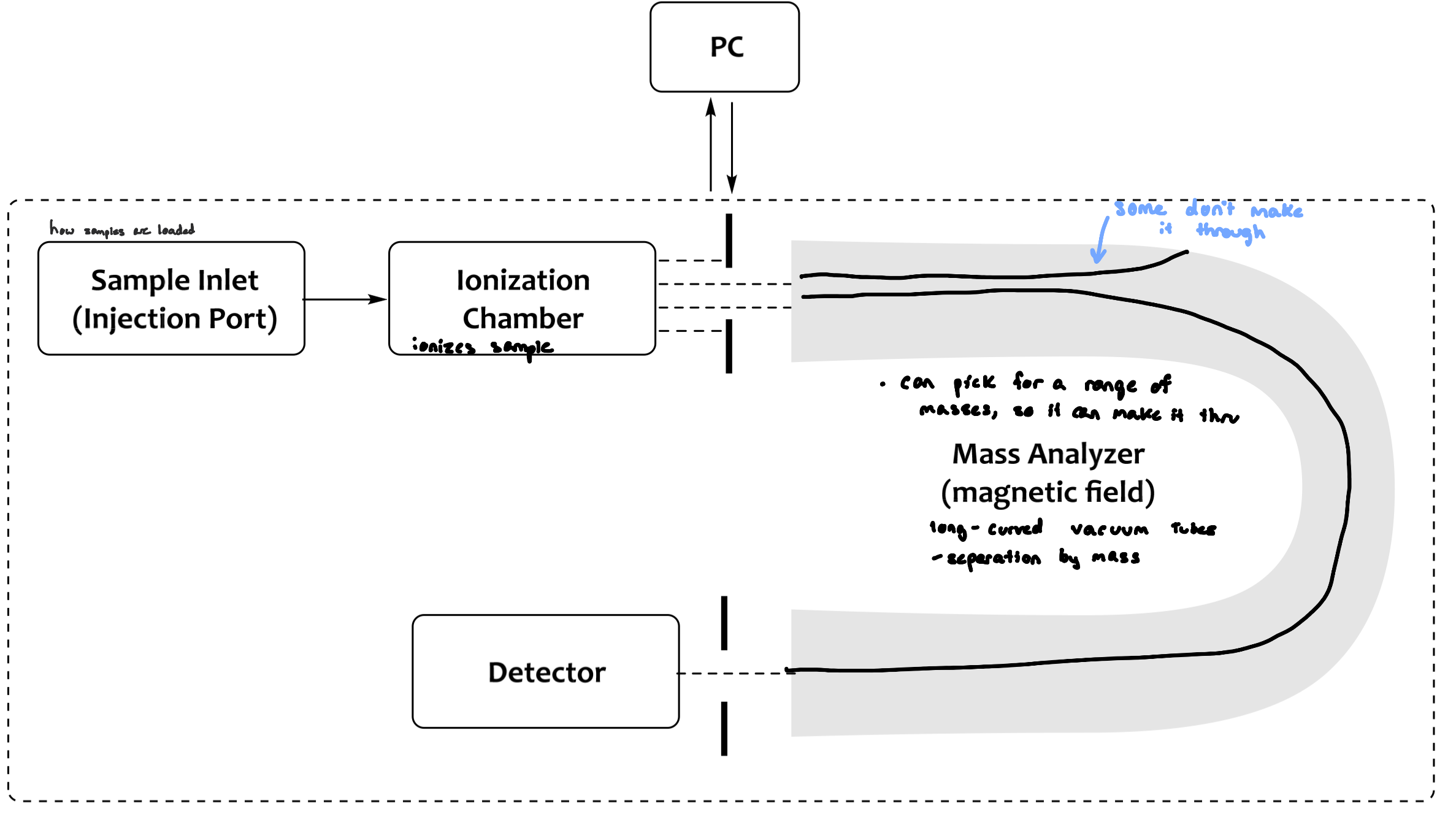
Sample Inlet
Pressure is atmospheric or vacuum depending on the next step
depends on type of ionization
The sample:
must be volatile at high temperatures
can’t put transition metals, their M.W is so heavy that it won’t transition from liquid to gas
temperature must not decompose sample
Ionization Chamber
the sample is introduced into the ionization chamber, at which point it is ionized (electron is dislodged, might be lone pair)
the method of ionization differs depending on the requirements and/or the compound of interest
ionization creates the molecular ion, which is unstable and can decompose
the most intense peak furthest to the right
decomposition creates daughter or fragment ions. These create a fingerprint of the compound
uncharged or neutral (don’t see them)
M=neutral molecule
M+•=molecular ion (radical cation)
e(-)=electron
x=daughter or fragment ions
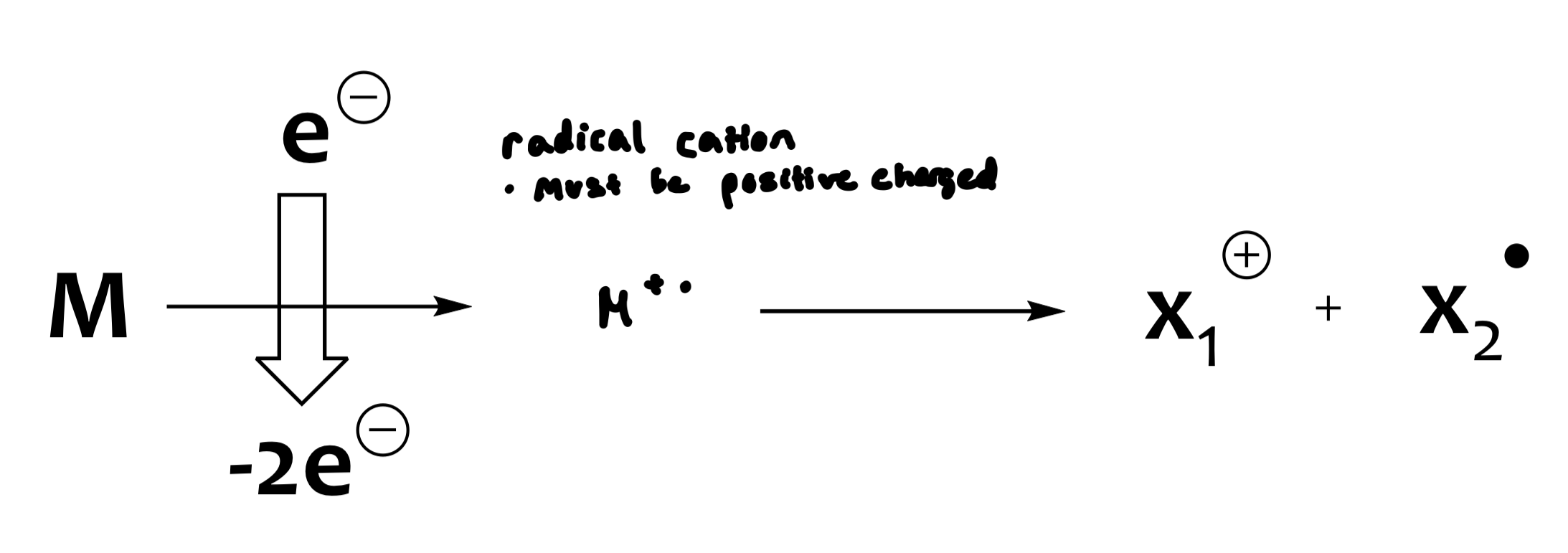
Electron Impact Mass Spectrometry (EI-MS)
large potential difference in accelerating plates (1-10 kV)
focusing slit provides a uniform beam of positive ions
ionization potential for most organic molecules (8-15 eV)
cheap and reliable method
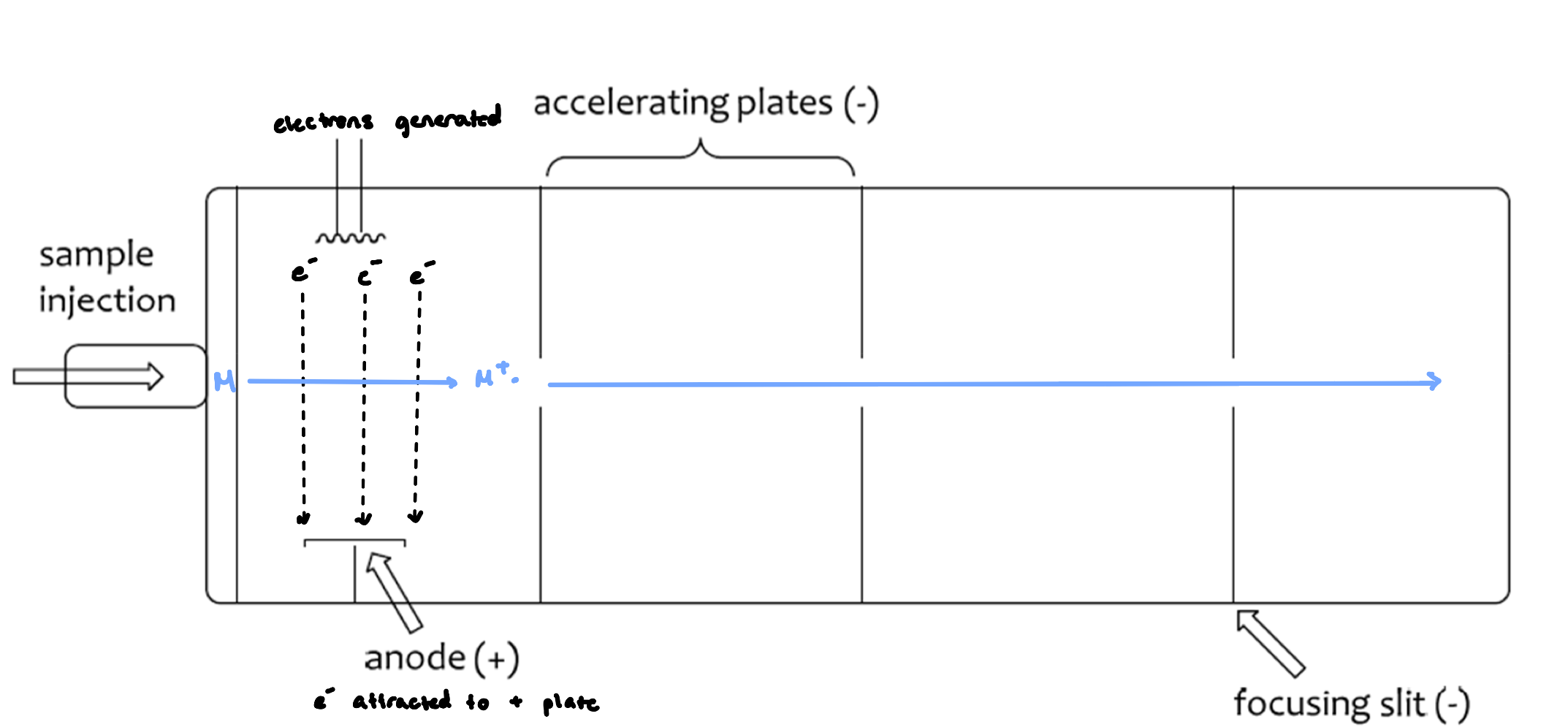
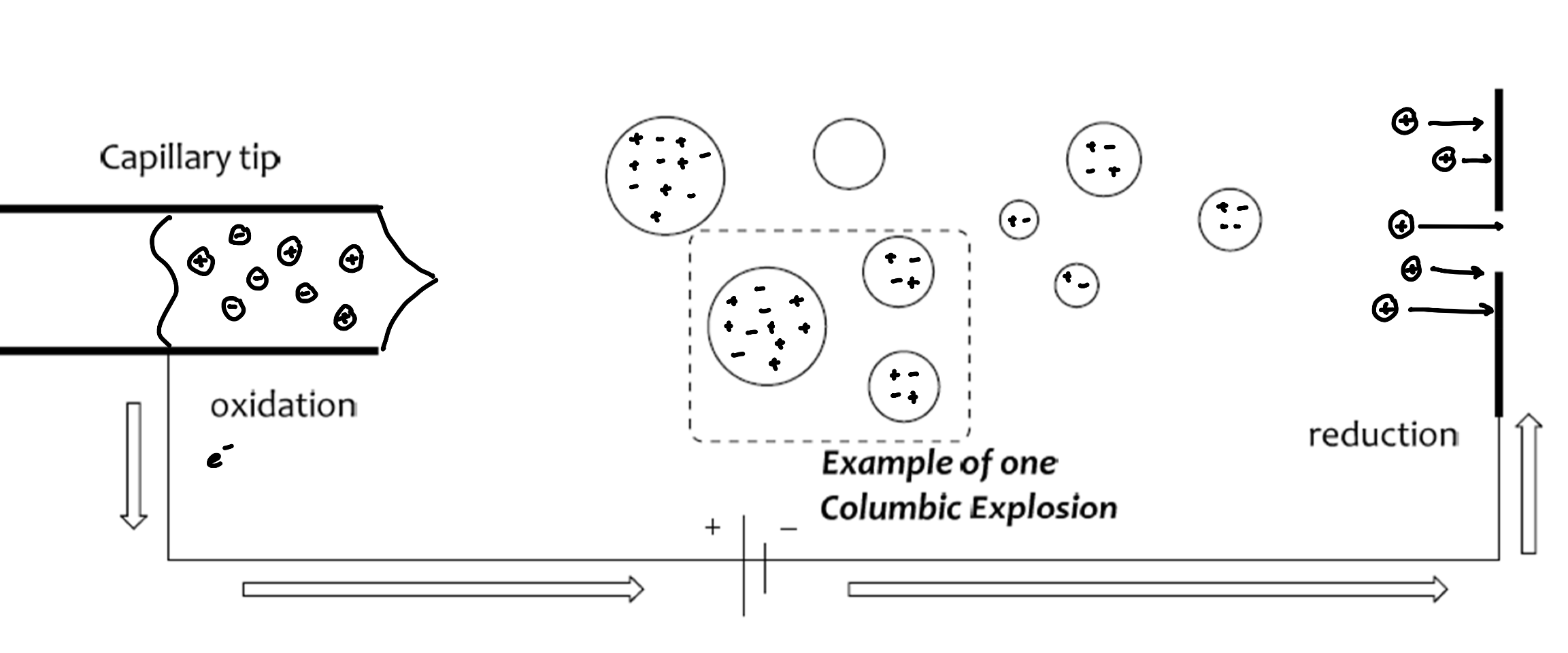
Electrospray Ionization (ESI)
for larger biomolecules
solvent evaporates out of the droplets, decreasing in size, increasing the positive character
as charges get closer, the droplets get smaller
results in Coulombic explosions
softer ionization technique (less harsh conditions)
more reliable ionization (per molecular ion)
Mass Analyzers and Detectors
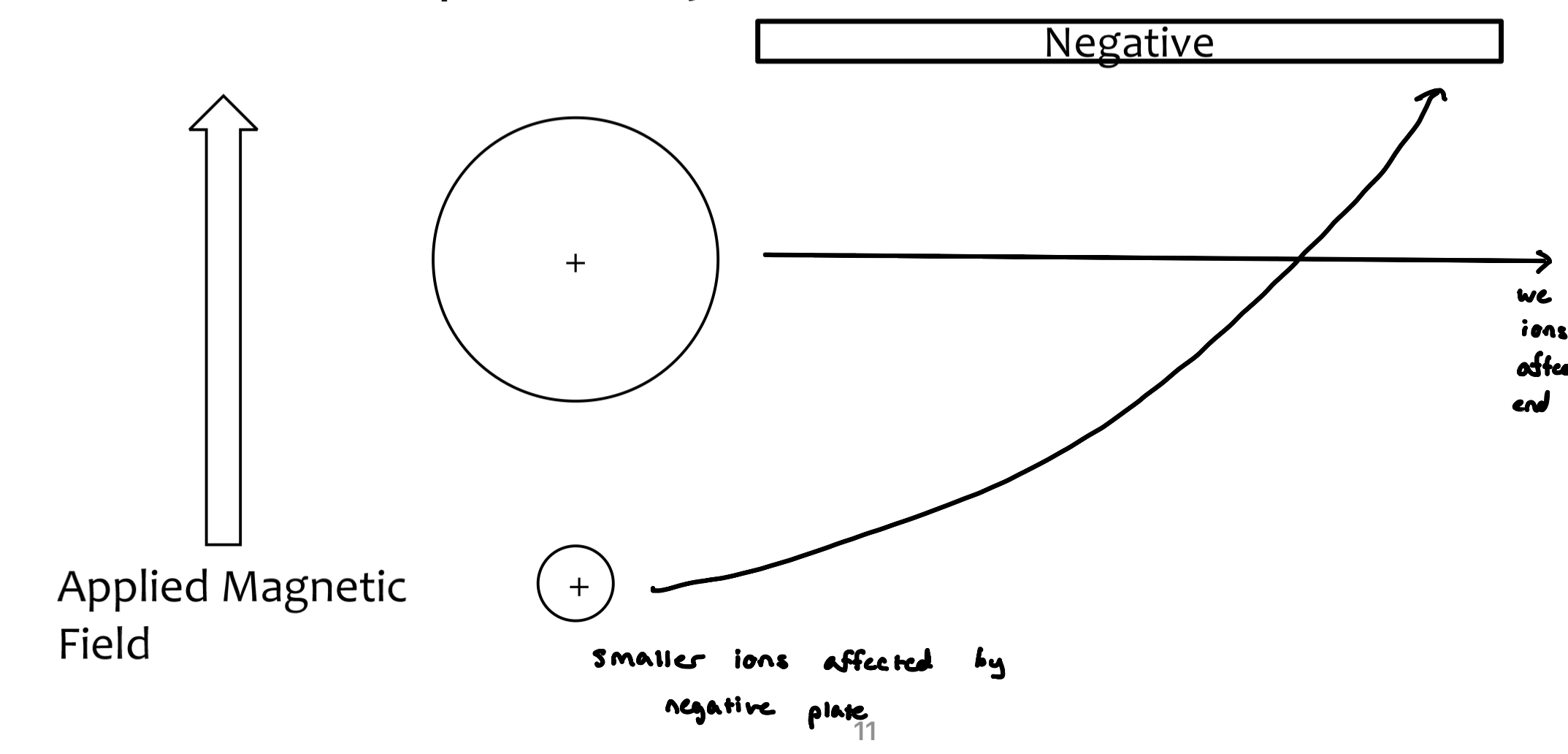
only cations are detected in mass spectrometry
neutral ions are not detected
anions can be detected depending on the ionization mode used
ions are separated based on their mass-to-charge ratio (m/z)
z=1
we don’t see larger ions because they don’t get affected by the negative plate and end up hitting the wall
Production of a Spectrum
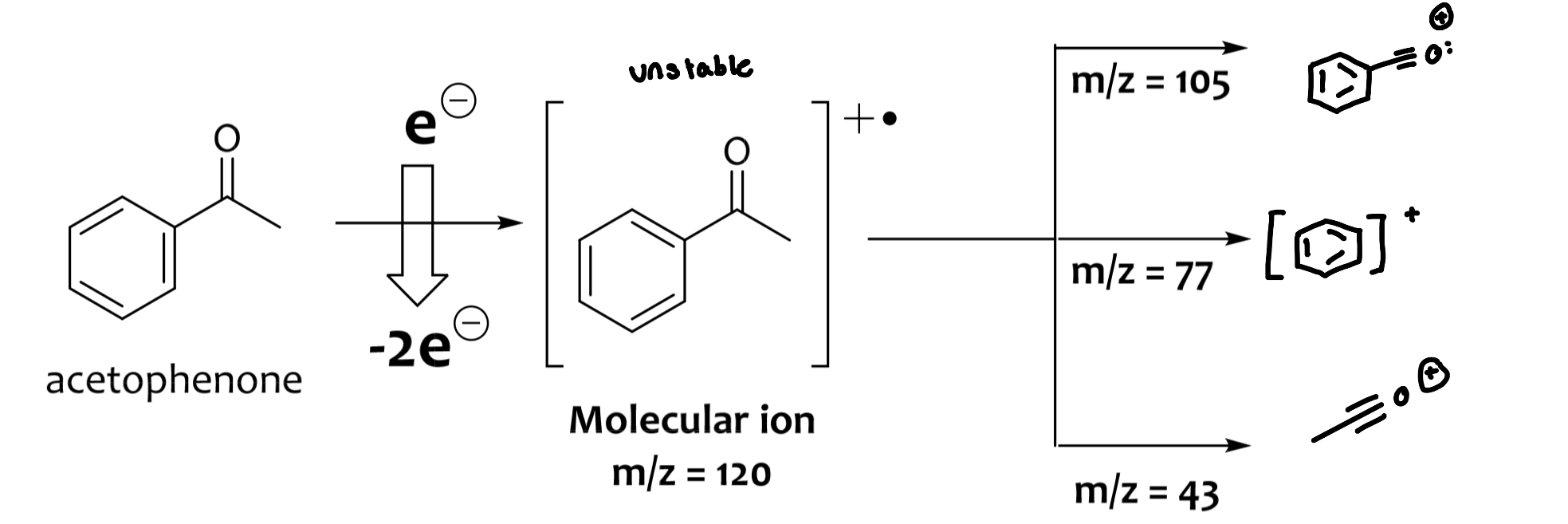
molecular ion peak can be the base peak
molecular ions and/or fragment ions travel through the mass analyzer, and collide with the detector
detector amplifies the signal
computers transform the signal into a spectrum
anything that’s not a molecular ion peak is a daughter fragment
Aromatic ring at 77m/z
M-15=loss of a methyl group
Fragmentation
molecular ions are unstable, and fragment to provide daughter/fragment ions
properly trained chemists can tease out a wealth of information from these fragmentation patterns
electrons in lone pairs > electrons in π-bonds > electrons in sigma bonds
HRMS and Exact Mass
HRMS provides an accurate measurement of mass (+0.00005%)
Isotopic Patterns in Mass Spec.
one amu above molecular ion peak = M+1
two amu above the molecular ion peak = M+2
what causes smaller peaks: isotopes
not generally important
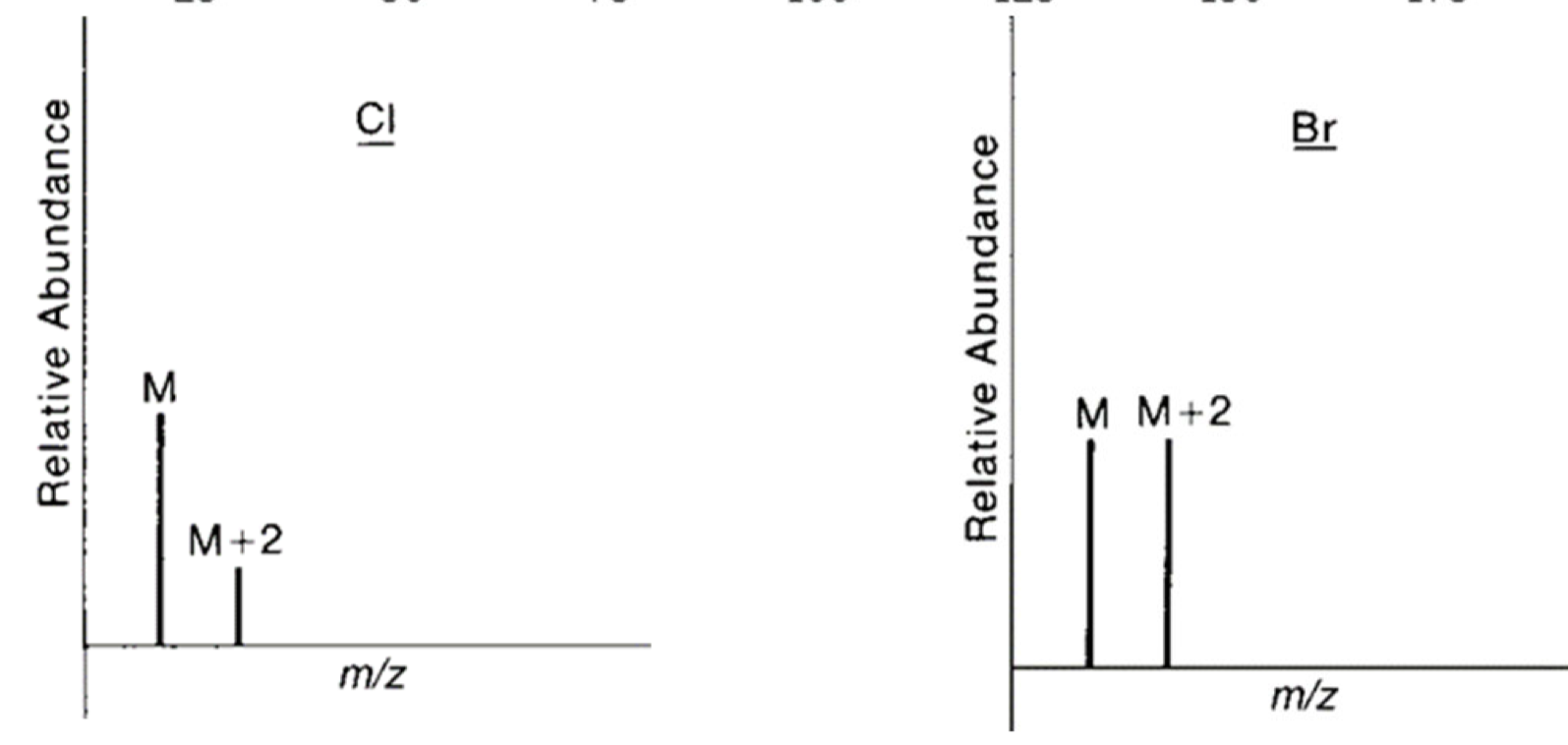
Cl and Br have significant isotopic peaks and are important in analysis
M+2 Peaks with Cl and Br
M+2 peak is important and diagnostic for Cl and Br
there is significant isotopic abundance of these heavier isotopes of both Cl and Br
the relative intensities between M and M+2 tell us whether Cl or Br is present
if M and M+2 are ~ 1:1 = Bromine
if M and M+2 are ~ 3:1 = Chlorine
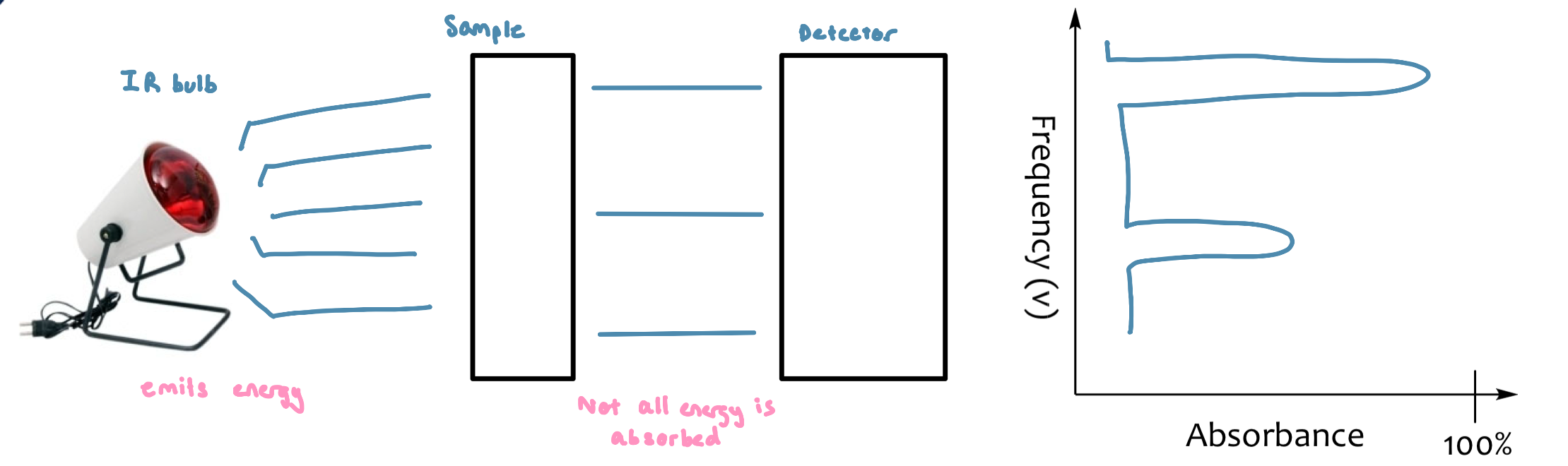
Spectroscopy
The study of the interaction between matter and electromagnetic radiation
electromagnetic radiation behaves like a wave and like a particle
particle component termed ‘photons’
Photon: a small, massless particle that contains a small wavepacket of EM radiation/light
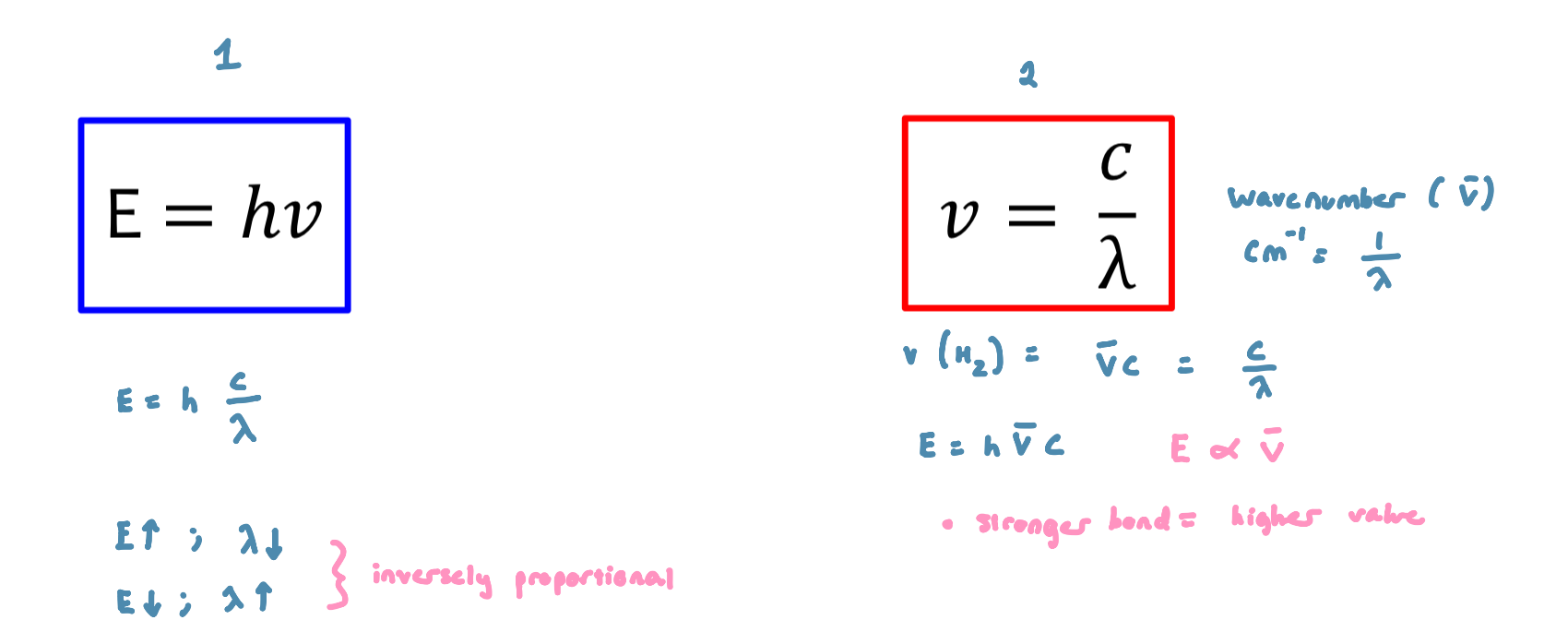
IR Absorption Figure
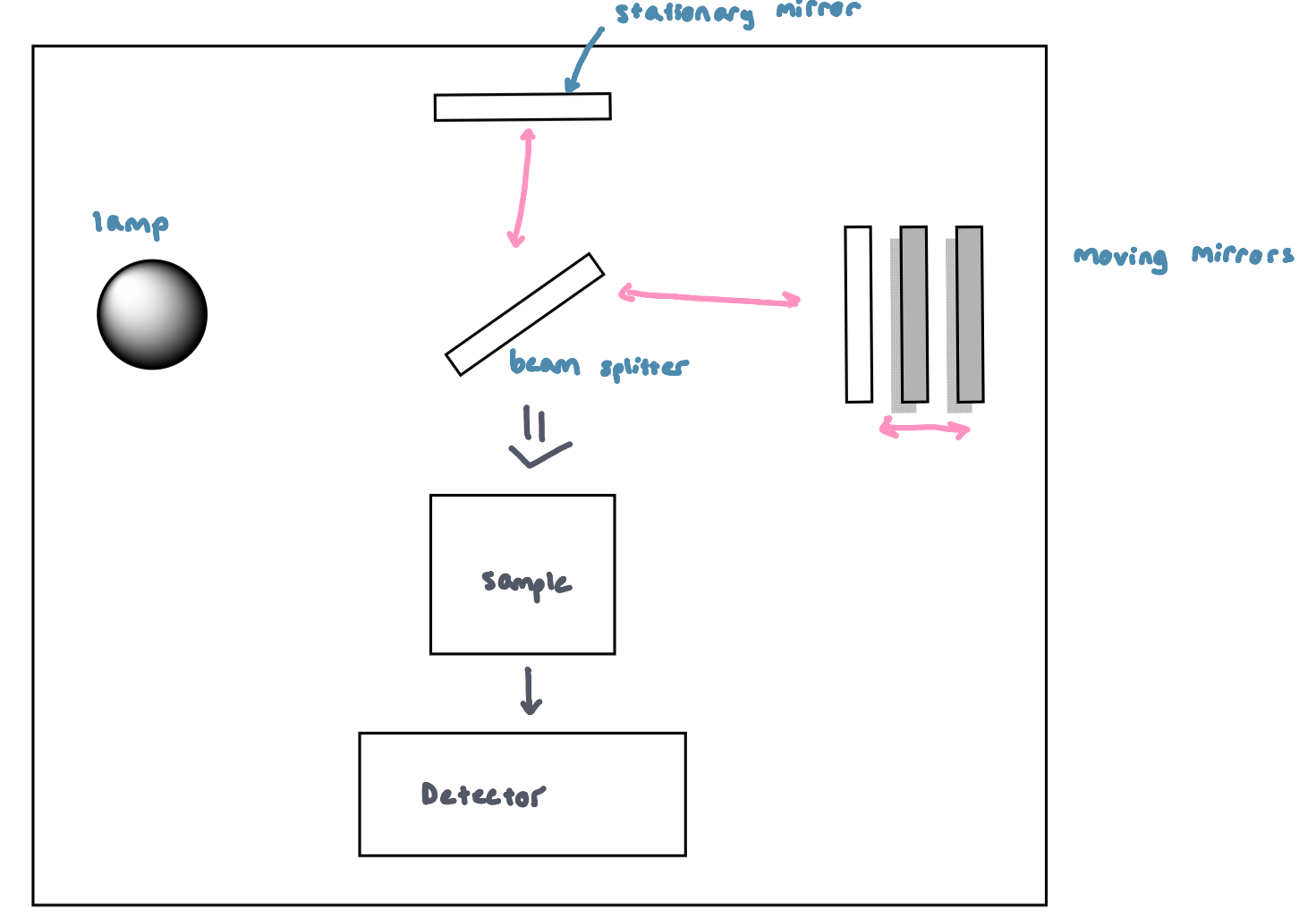
Fourier Transform IR (FTIR) Figure
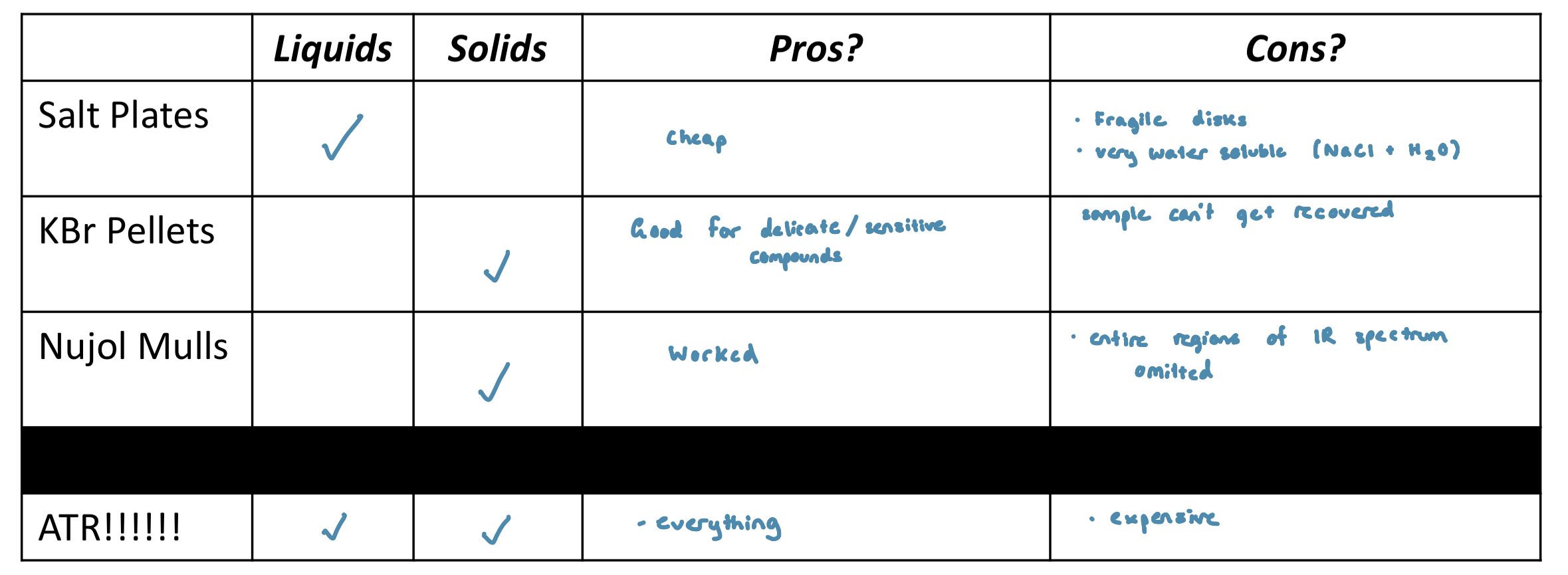
Sample Preparation: Problem, Glass and Plastics Absorb strongly in the IR region
Molecular Vibrations
the quantum mechanical energy levels observed in IR spectroscopy, which we perceive as heat
absorption of energy changes vibrational state
covalent bonds naturally oscillate in a way that resembles an oscillating spring
As a covalent bond oscillates, the dipole moment of a bond (molecule) will change as well
This change in dipole moment causes an EM field to be produced
the weaker the dipole moment, the weaker the field
the stronger the dipole moment, the stronger the field
molecules only absorb select frequencies. These frequencies match the natural vibrational frequencies
How can electromagnetic (EM) radiation interact with matter?
As atomic particles exhibit both wave and particle properties, EM radiation interacts with matter in 2 ways:
collision
coupling: wave property of the radiation matches the wave property of the particle, the waves couple to the next quantum energy level
Vibrational Modes: Stretching Vibrations
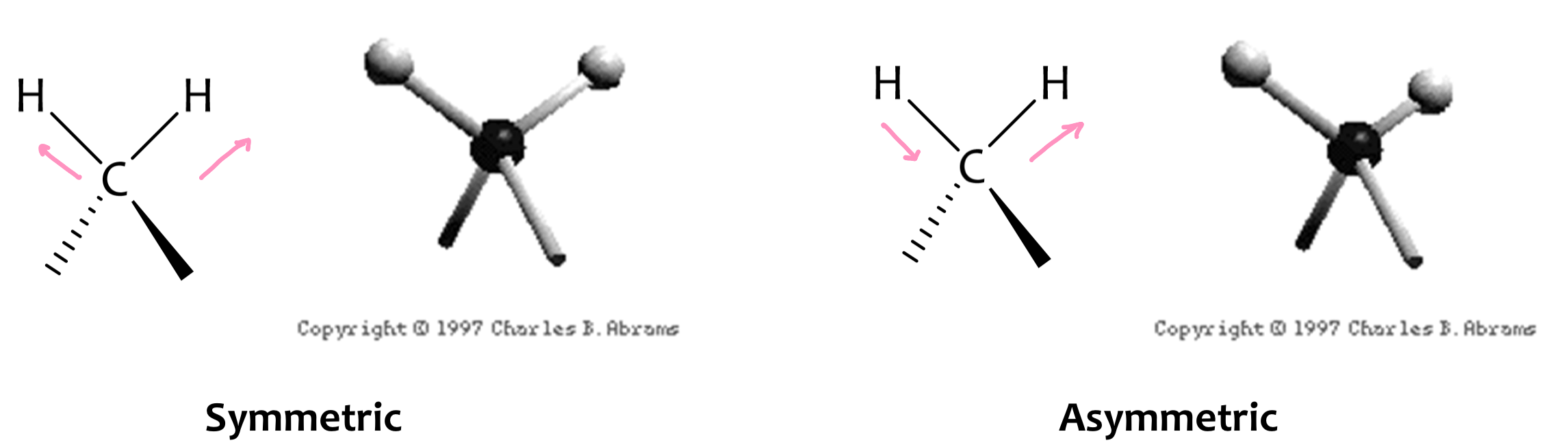
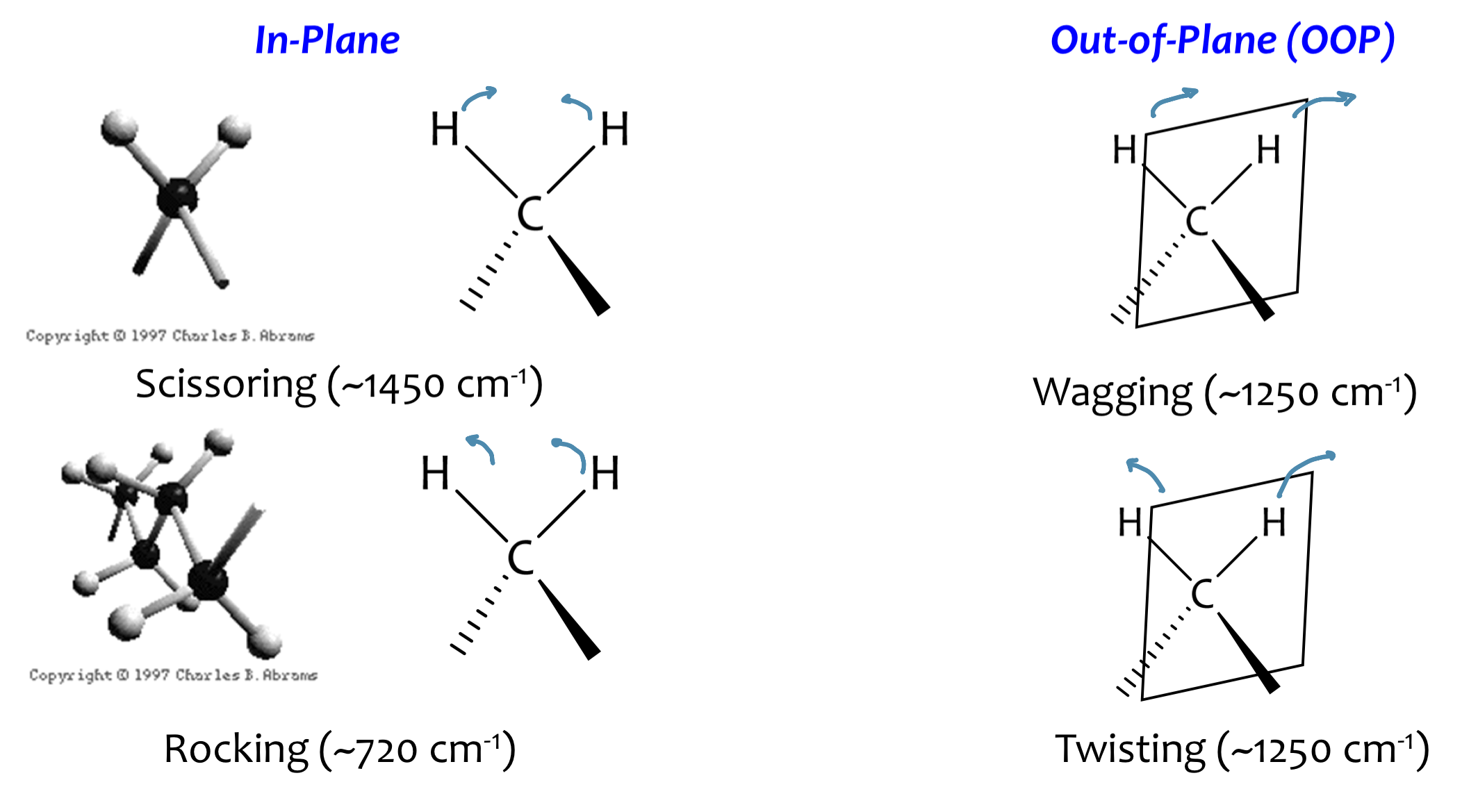
Vibrational Modes: Bending Vibrations
Bond Properties
the force (F) needed to extend or compress a spring by some distance (X) is proportional to that distance
F=kX, k is a force constant that is representative of the stiffness of the spring
chemical bonds are like springs
the stronger the bond, the greater the force constant (k)
bonds between atoms of larger masses (µ) vibrate at lower frequencies
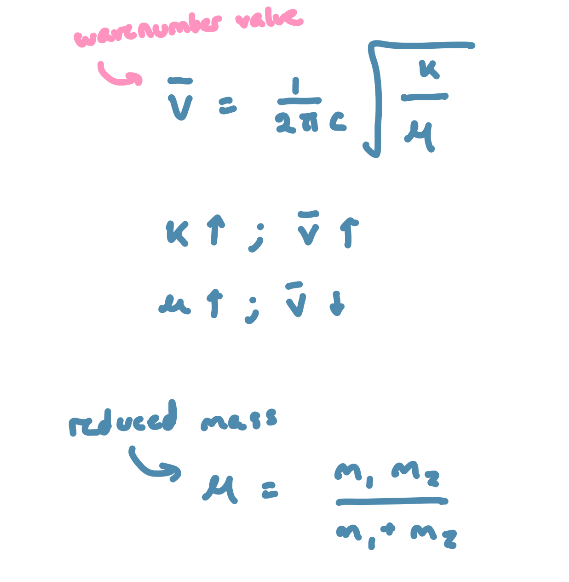
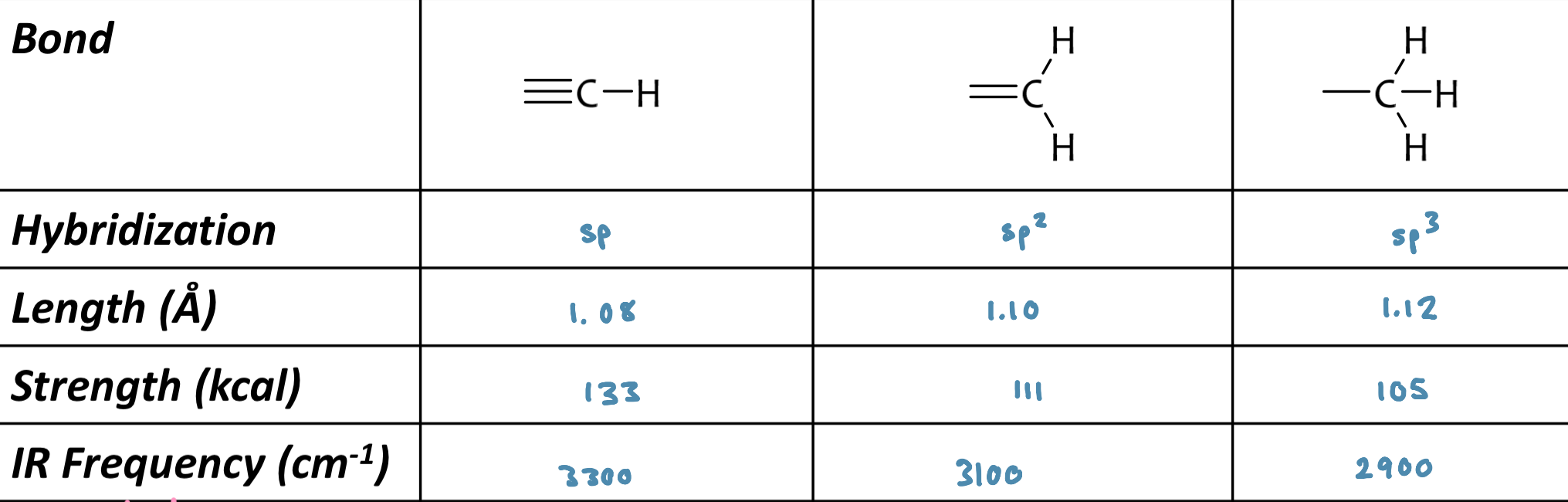
Bond Strength
sp CH > sp2 CH > sp3 CH
Absorptions of CH and CC bonds
frequency of absorption is a function of hybridization (tells us bond strength)
CH stretching
less than 3000 cm-1 usually indicates saturated sp3 CH
in between 3000 cm-1 to 3150 cm-1 usually indicates sp or sp2 (vinyl or aromatic) CH
stronger bond = higher absorption
exceptions to the rules:
Aldehydic: two bands 2900-2750 cm-1
Cyclopropyl: ~3100cm-1, strong absorption because of ring strain
Alkenes
Characteristic regions:
sp2 CH stretch ( >3000cm-1)
sp2 CH oop (1000-650cm-1)
C=C stretch (1660-1600cm-1)
Conjugation effects: have lower wavenumbers
OH stretch
concentrated ~3300 cm-1 (broad)
~3600cm-1 (sharp) : dilute
intramolecular hydrogen bonding will weaken the OH stretching vibration
more concentrated=more H-bonding=blob peak
Amines
N-H stretching located in the same region as OH stretching
substitution of the amine can be inferred from the observed absoprtions
don’t see 3º amine, it has no H
Nitrile
characteristic medium intensity absorption in the triple bond region
C≡N: ~2250 cm-1
CN stretch: 1000-1350cm-1
Pitfalls:
C≡C bond: located near C≡N
conjugation
Carbonyl compounds
Characteristic absorption from 1650-1850cm-1
depends on structure the carbonyl is bound to
Factor 1: EWG and ⍺-haloeffect
groups which remove e- from the C=O will manifest as a higher frequency of absorption
Factor 2: Conjugation (resonance)
carbonyls that are conjugated will show lower frequency of absorption when compared with an unconjugated molecule
Factor 3: H-bonding
predominant in carboxylic stretch
weakens the C=O bond stretch (lowering force constant (k))
gives lower absorption
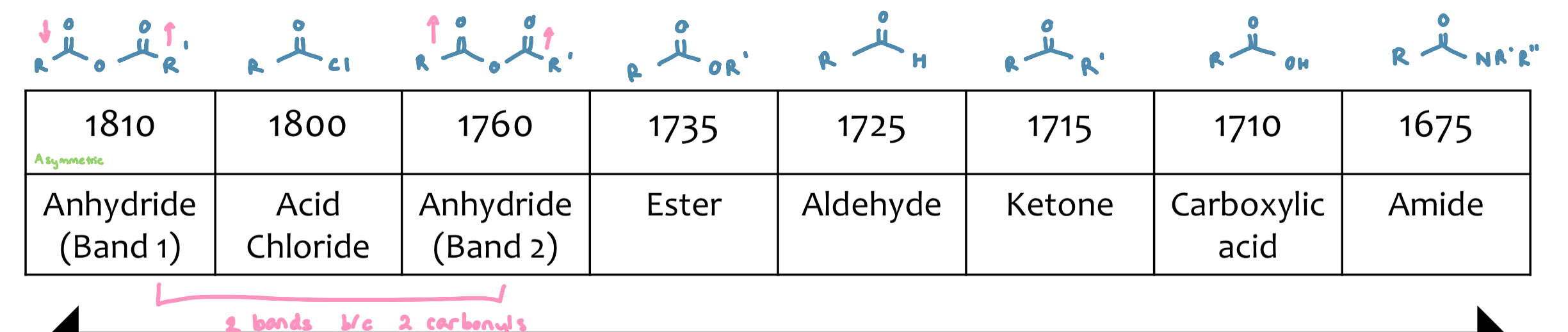
Aldehydes
Characteristic regions
1725-1740 cm-1
pair of weak bands in CH (only seen when not obscured by CH region)
2800-2860cm-1
2700-2760cm-1
Esters
need to be careful with our conjugation arguments
if the carbon chain on the ether O can have resonance (ending up with a negative charge on the carbon) it will pull e - density and strengthen the absorption
Deuterated Solvents
deuterated solvents are used prepare NMR samples for analysis
the 1H nuclei are NMR active. 2H (or D) are not NMR active
Nuclear Spin
any atomic nuclei with either an odd mass or odd atomic number, or both will possess a quantized atomic spin
the number of spin states allowed is defined:
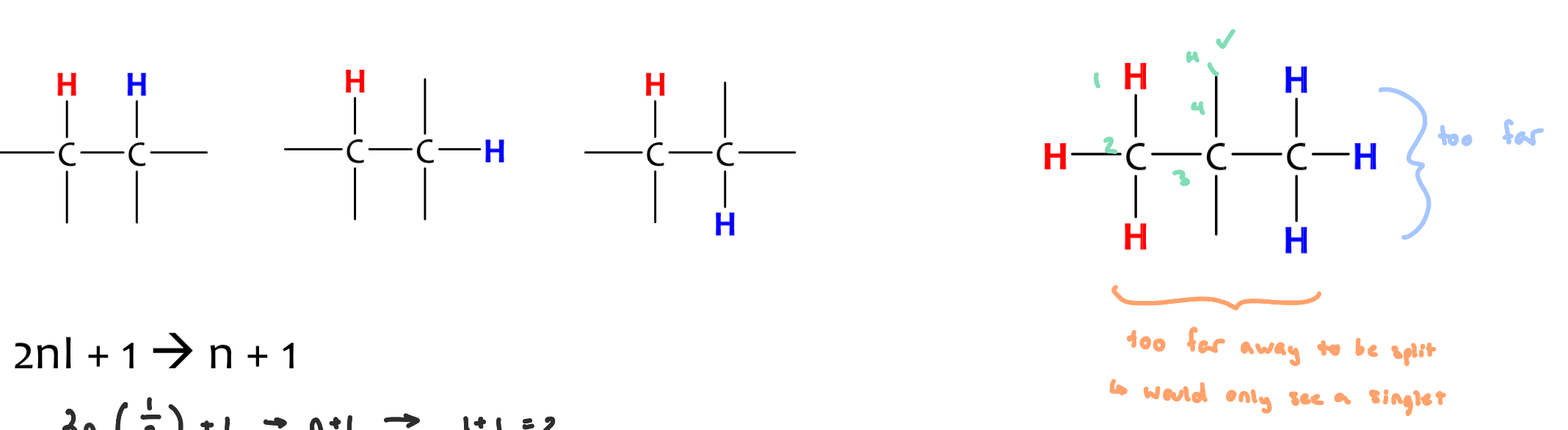
2nI + 1
n = nuclear spin of interest
I = physical constant
so, if we are interested in knowing the number of possible spin states for 1H (nuclear spin quantum number = 1/2)
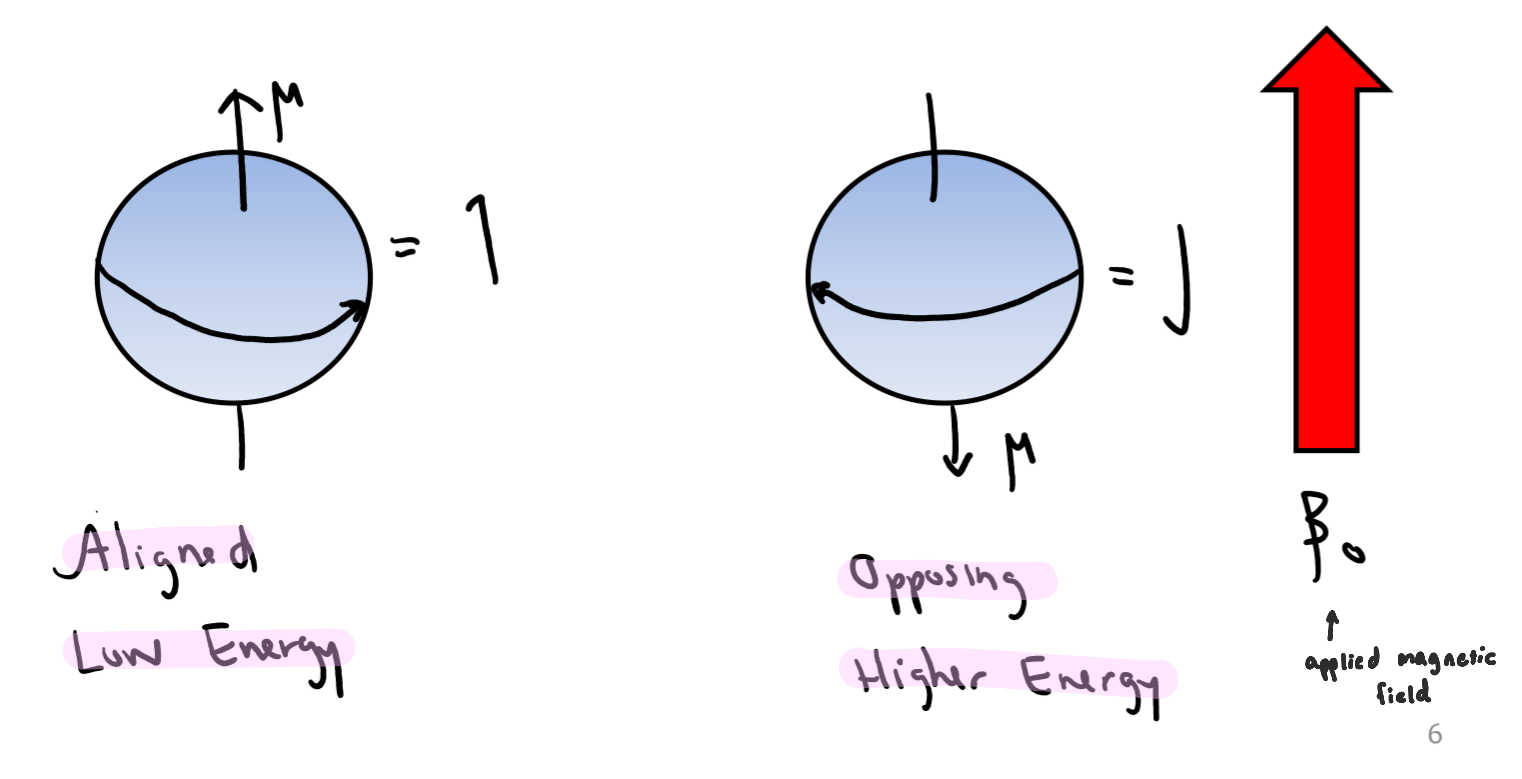
Nuclear Spin in an Applied Field
when a charged particle (the nucleus) is spinning, it creates a magnetic field (µ)
spin states are equivalent in energy in an applied magnetic field
Magnetic Fields

⍺ and β Spin States
If the normally disordered magnetic moments are exposed to an external magnetic field, they will align into two forms
alpha (⍺) – aligned with magnetic field (β0)
beta (β) – opposing β0
when an external magnetic field is applied (β0), the degenerate spin states split into two states of unequal energy
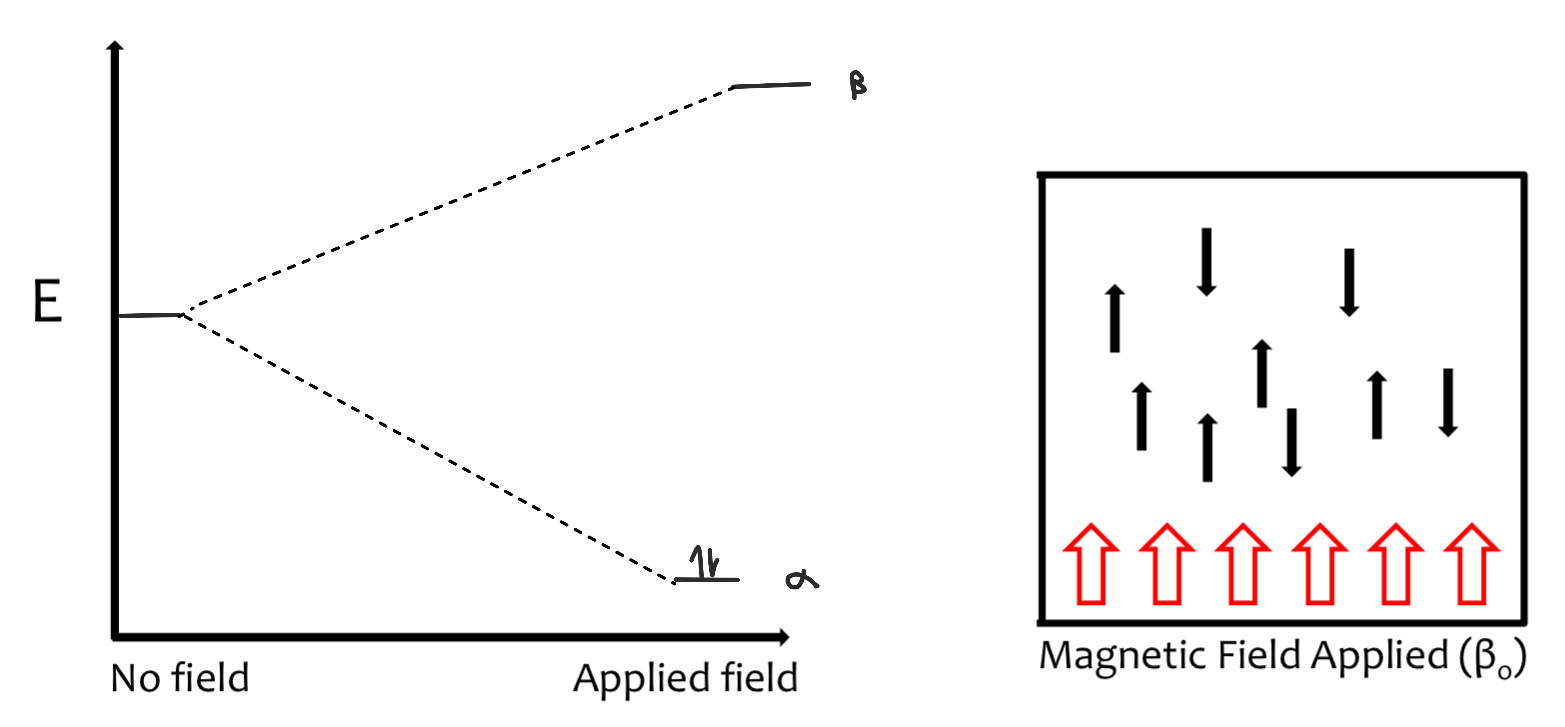
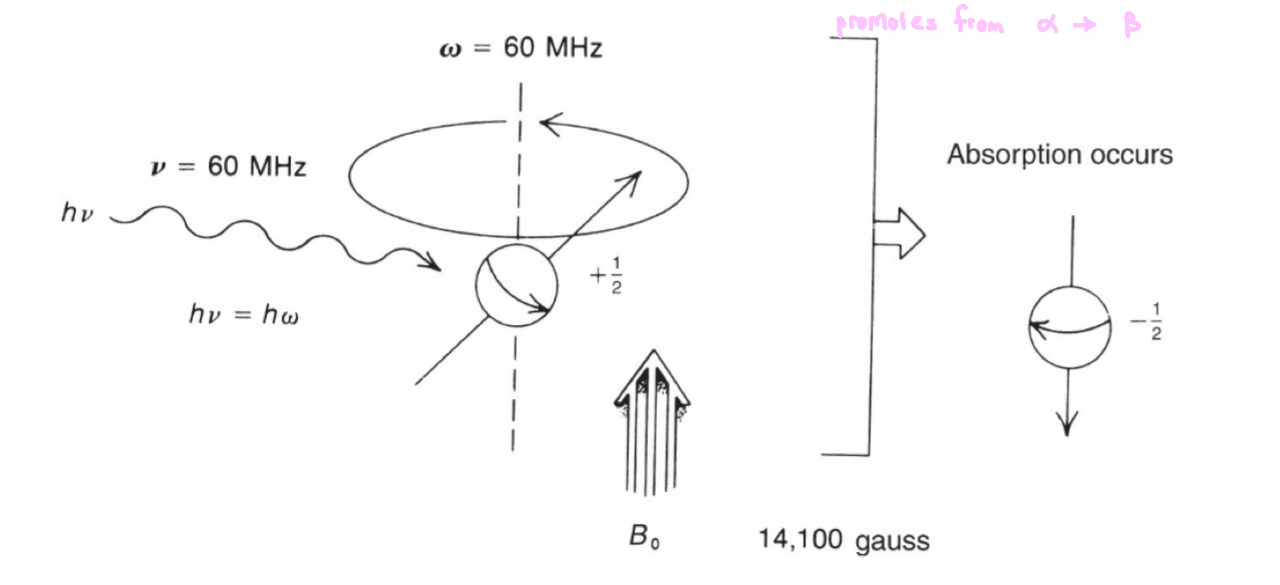
Energy Absorption
energy absorption is a quantized phenomenon
E(absorbed) = (E-1/2 state - E +1/2 state) = hv
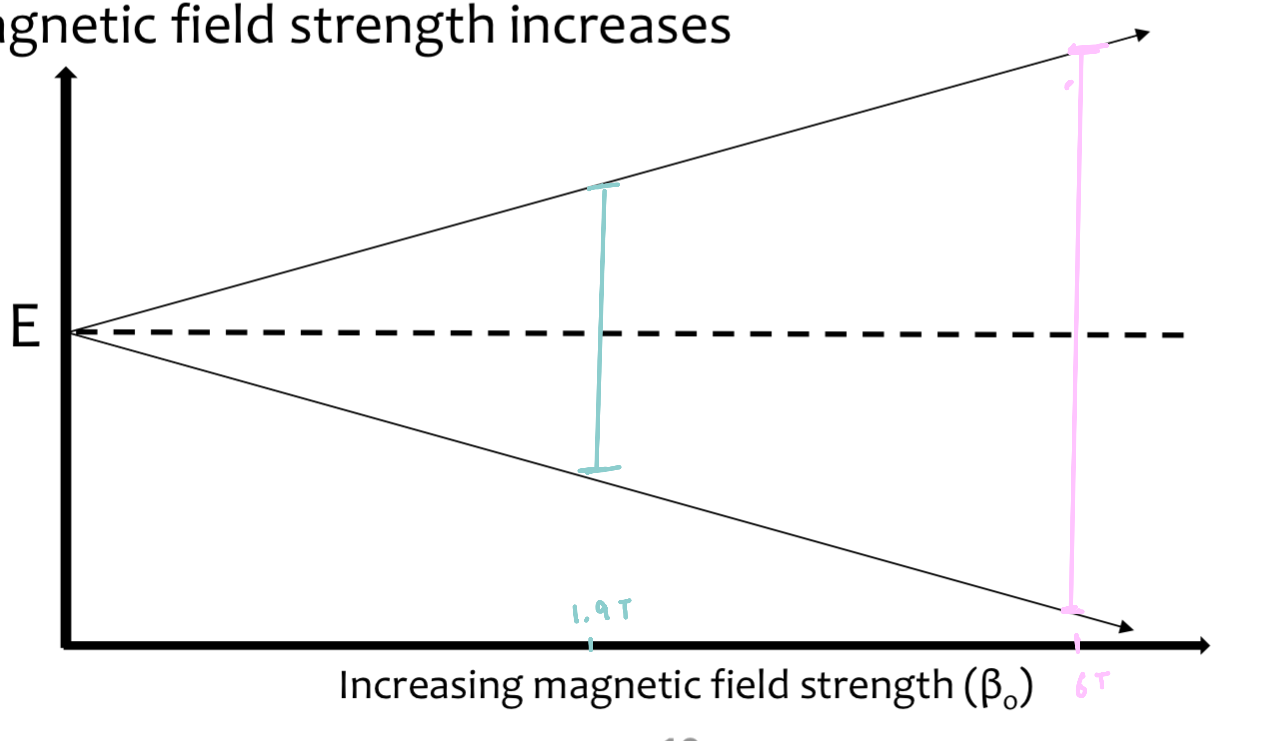
the energy gap between each state increases as the applied magnetic field strength increases
Larmor Frequency
in the presence of an applied magnetic field, a spinning nucleus begins to wobble
a nucleus that precesses about its own axis, which also possesses angular frequency
this is called the Larmor frequency (ω)
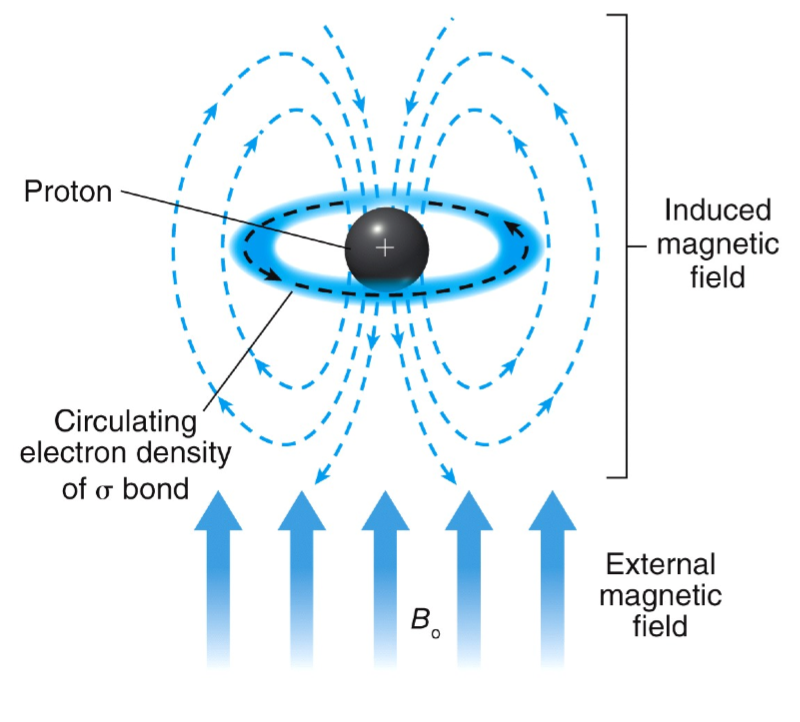
Chemical Shift (∂)
electrons swirl around the central nucleus
in an applied magnetic field, valence electrons are caused to circulate
this generates a counter magnetic field which opposes the applied field
lower energy difference between spin states
lower energy requires to flip spin states
13C NMR – Chemical Shift
the more substituted a carbon is, the more deshielded it becomes
increasing electronegativity of nearby groups, the more deshielded
increasing the number of nearby electronegative groups, the more deshielded
decreasing distance between the carbon nuclei and nearby electronegative groups, the more deshielded
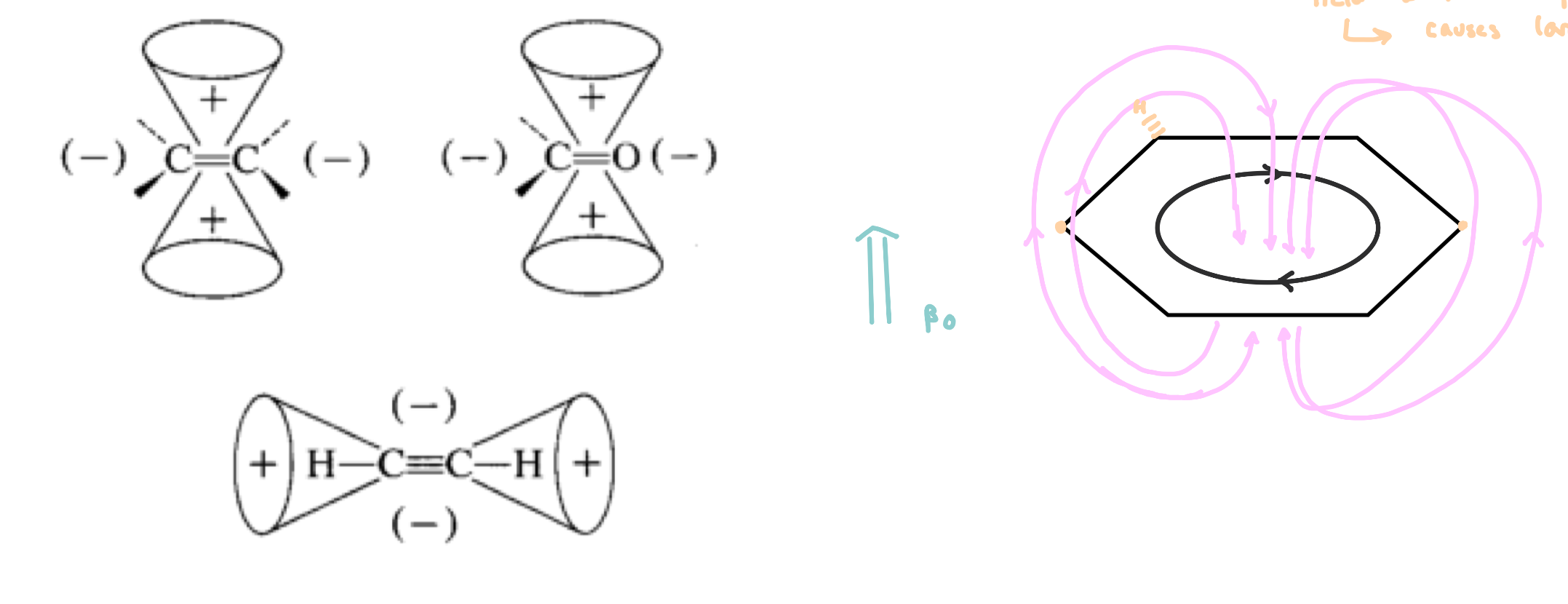
Chemical Shift – Anisotropy
Anisotropy: non uniform application of an electric field
presence of EWG will remove electron density
in the presence of an applied magnetic field, the electrons in π bonds begin circulate, causing an induced magnetic field
areas are more shielded and more deshielded than expected
e.g. Benzene: carbons are near the induced magnetic field with the applied magnetic field
causes a large deshielding event
Function of TMS
NMR active nuclei will couple with nearby NMR active atoms
to avoid this we use TMS (tetramethylsilane)
TMS serves as a reference compound
13C NMR – Number of Signals
12C is the most abundant isotope of carbon (not NMR active)
13C has a natural abundance of ~1.1%
our starting point for spectral interpretation
the number of signals is determined by the number of non-equivalent carbon atoms present in the molecule
equivalent carbon nuclei are in the same chemical and magnetic environment
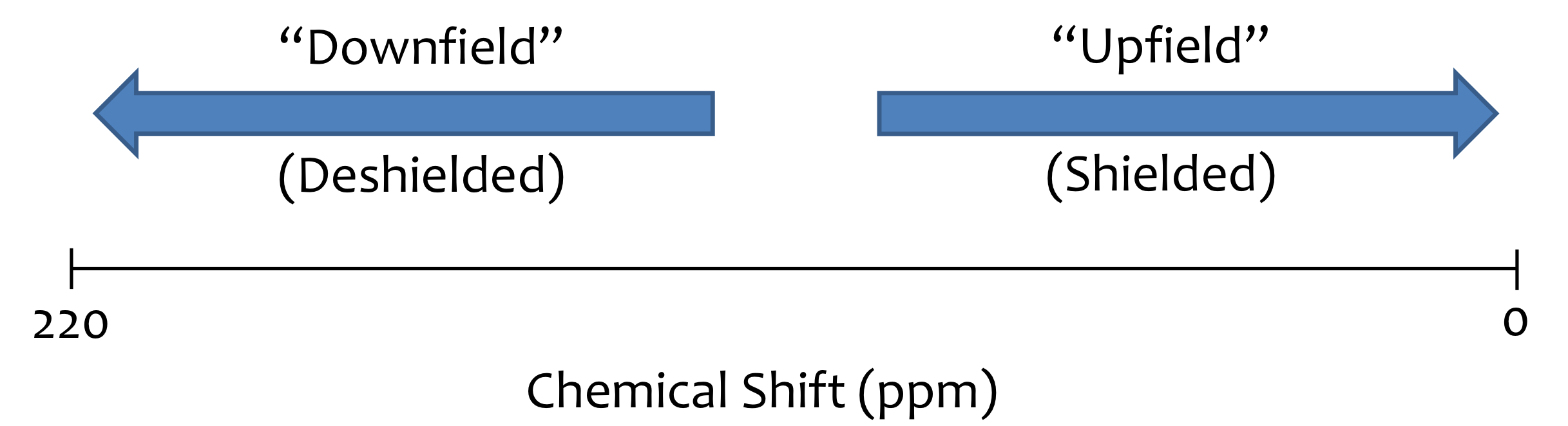
Downfield vs Upfield
DEPT NMR
the result is a 13C NMR spectrum were signals display different phases depending on the number of hydrogen atoms attached
DEPT 135
Positive: CH3, CH
Negative: CH2
Importance: odd H’s up, even H’s down
DEPT 90
Positive: CH
Negative: N/A
Importance: only shows methine (only CH Carbons)
DEPT 45
Positive: CH3, CH2, CH
Negative: N/A
Importance: Quaternary carbons do not appear
1H NMR
chemical shift: shielded or deshielded
number of signals: how many non-equivalent protons
integrals/integration: how many protons are present
spin-spin splitting: are they seeing other protons
J-Coupling constants: distance between peaks (in Hz) of a particular signal?
spectral window from 0 to 10ppm
Integrals & Integration
method to quantify the relative number of equivalent protons
divide each integration value by the smallest one to get the ratio
the user sets the ratio (multiply the ratio if needed)
Spin-Spin Coupling
More complicated patterns exist when coupling constants of neighboring nuclei are different
multiplet= (mult.)
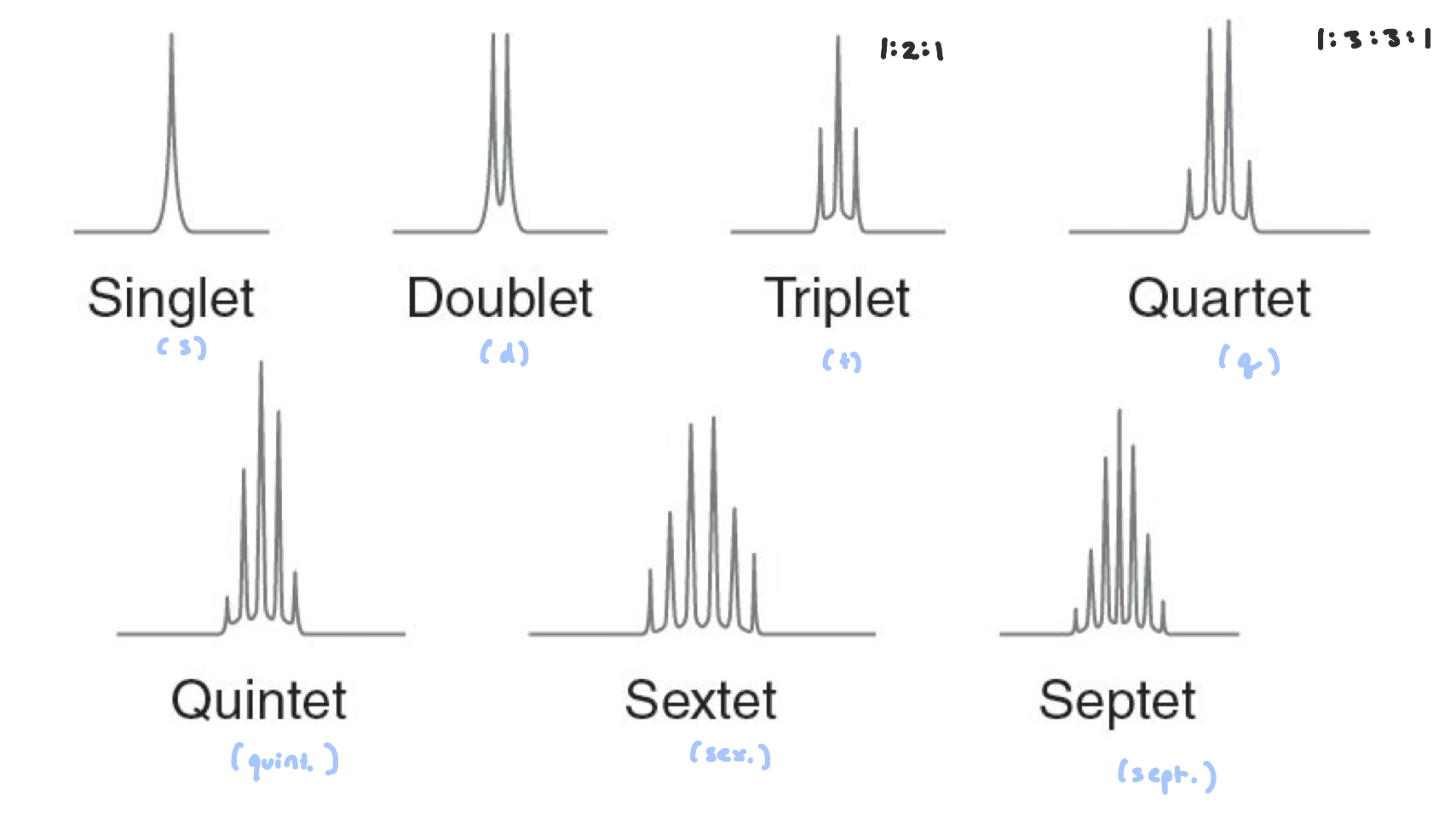
Signal Splitting
equivalent protons cannot split each other
to split each other, H’s need to be 2-3 bonds away from one another
n+1
H’s part of a tert-butyl group show up as singlet
Pascal’s Triangle
splitting patterns follow similarly to Pascal’s Triangle
the number of signals a nuclei will exhibit depends on the number of active nuclei it is surrounded by (2-3 bonds away)
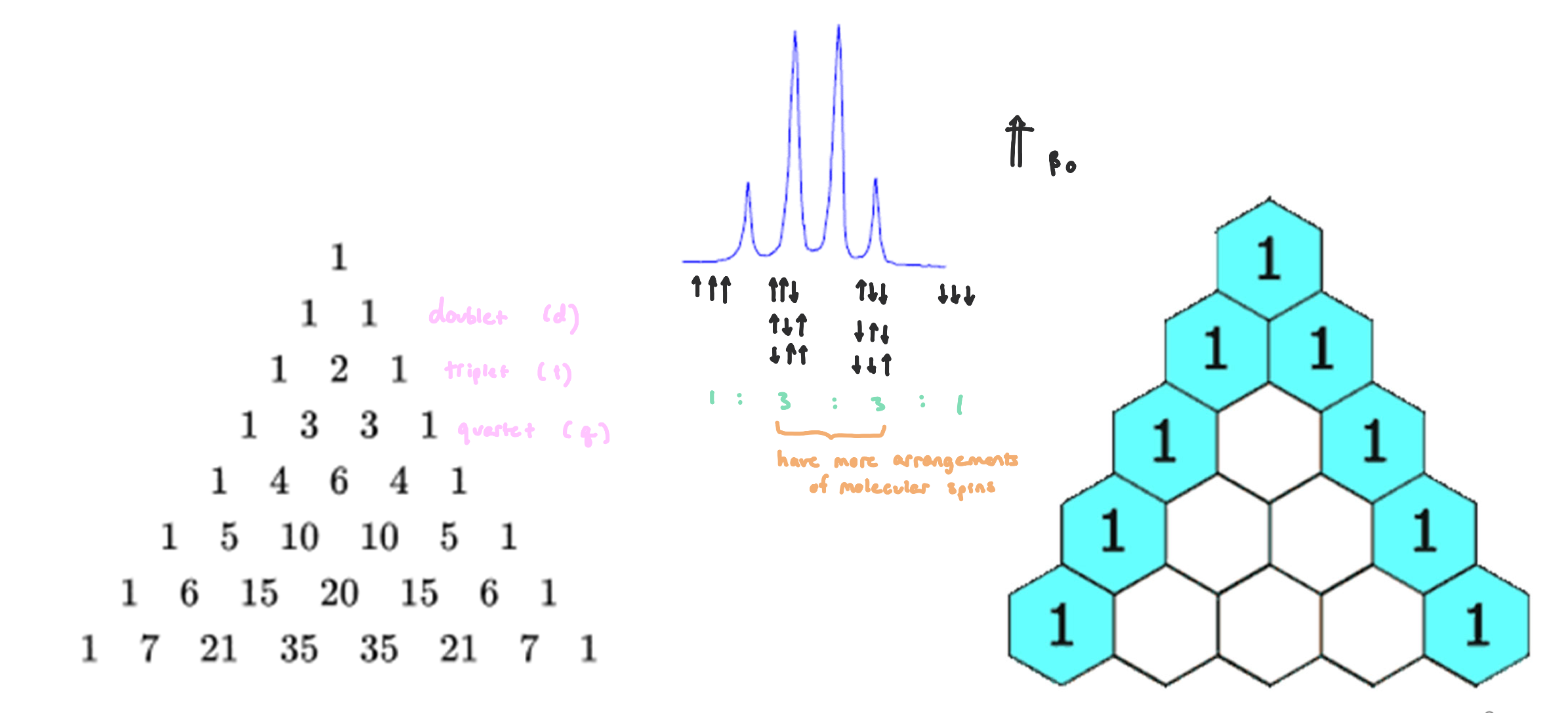
J-Coupling Constants
protons influence other protons creating different environements
affects the splitting pattern
affects the size of the spliting
our primary concern would be 3JHH for most protons and 2JHH for alkenes
dependent on the MHz of the instrument used
difference in ppm x MHz of the instrument = Jcoupling
Exchangeable Protons
X-H protons will exchange via hydrogen bonding
X-H protons do not split
often appear as broadened peaks
Alkenes
The protons can’t rotate due to the double bond, and the splitting pattern is like a 1:1 doublet splitting pattern with some space in between
is more deshielded when part of the alkene