Nomenclature
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
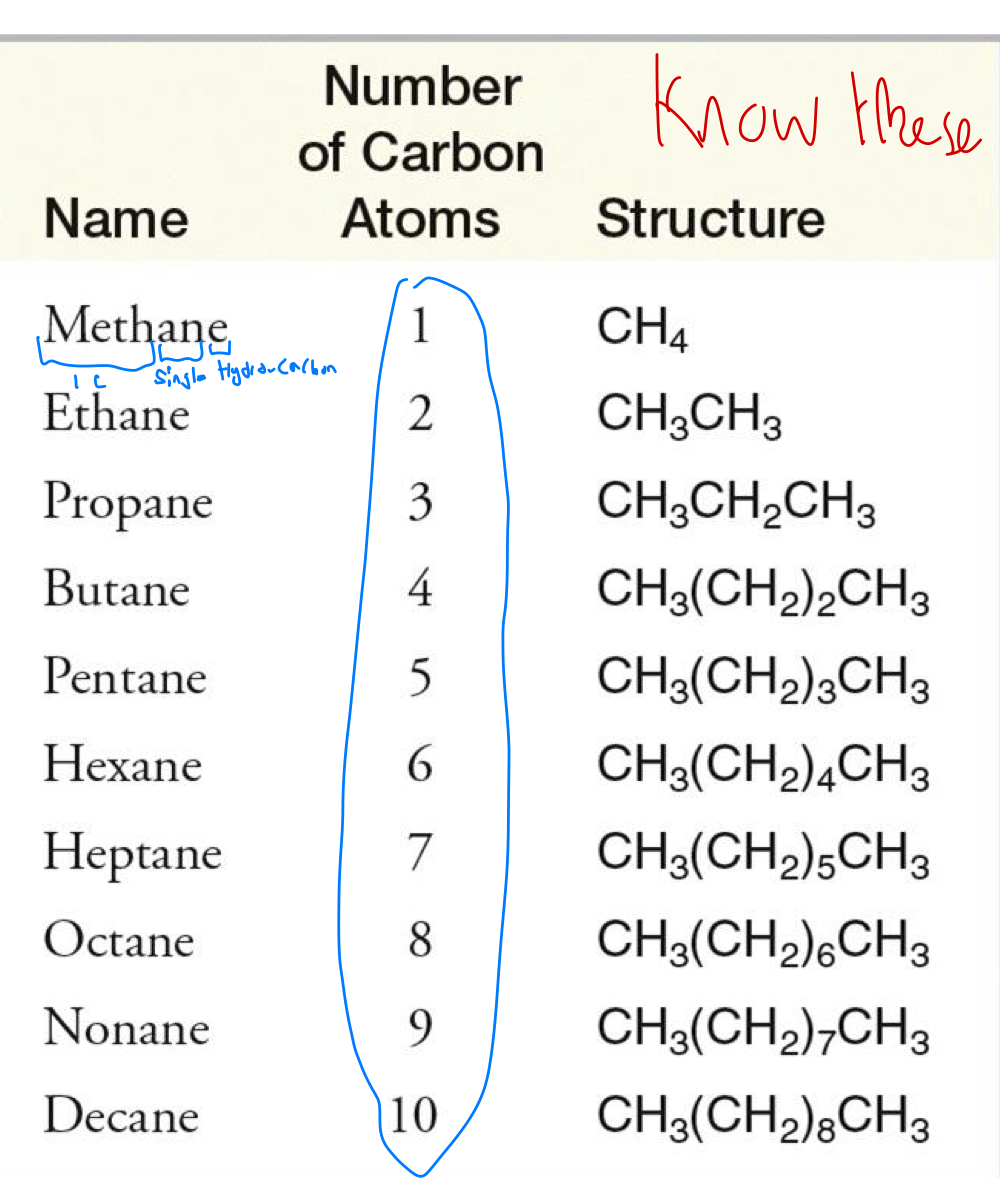
naming unbranched alkanes
prefix: based on table
infix: alkane or alkene or alkyne
Suffix: e for hydrocarbon, ol for alcohol
naming hydrocarbon branches and benzene branch name
alkyl groups, so if its a chain on 1, then its methyl, 2 is ethyl, etc
for benzene as a branch: its phenyl
naming branched alkanes
longest chain is parent chain
number from closest to branch for lowest number
location of the branch - name of brance name of parent chain
more than one identical branches alkane
number carbons so that lowest number to first encountered branch
if there is multiple of the same branch use di, tri, tetra, penta, hexa, etc
comma to separate position number
EX: 1,2 - dimethylhexane
different branches alkane
List in ABC order
give lowest number to branch encountered first
if same position, give first in alphabetic one, lower number (dont include di, tri, etc for alphabetic order)
ex: 1 - ethyl - 2,2 - methylhexane
choosing parent chain when there are different options
choose the one with most substituents.
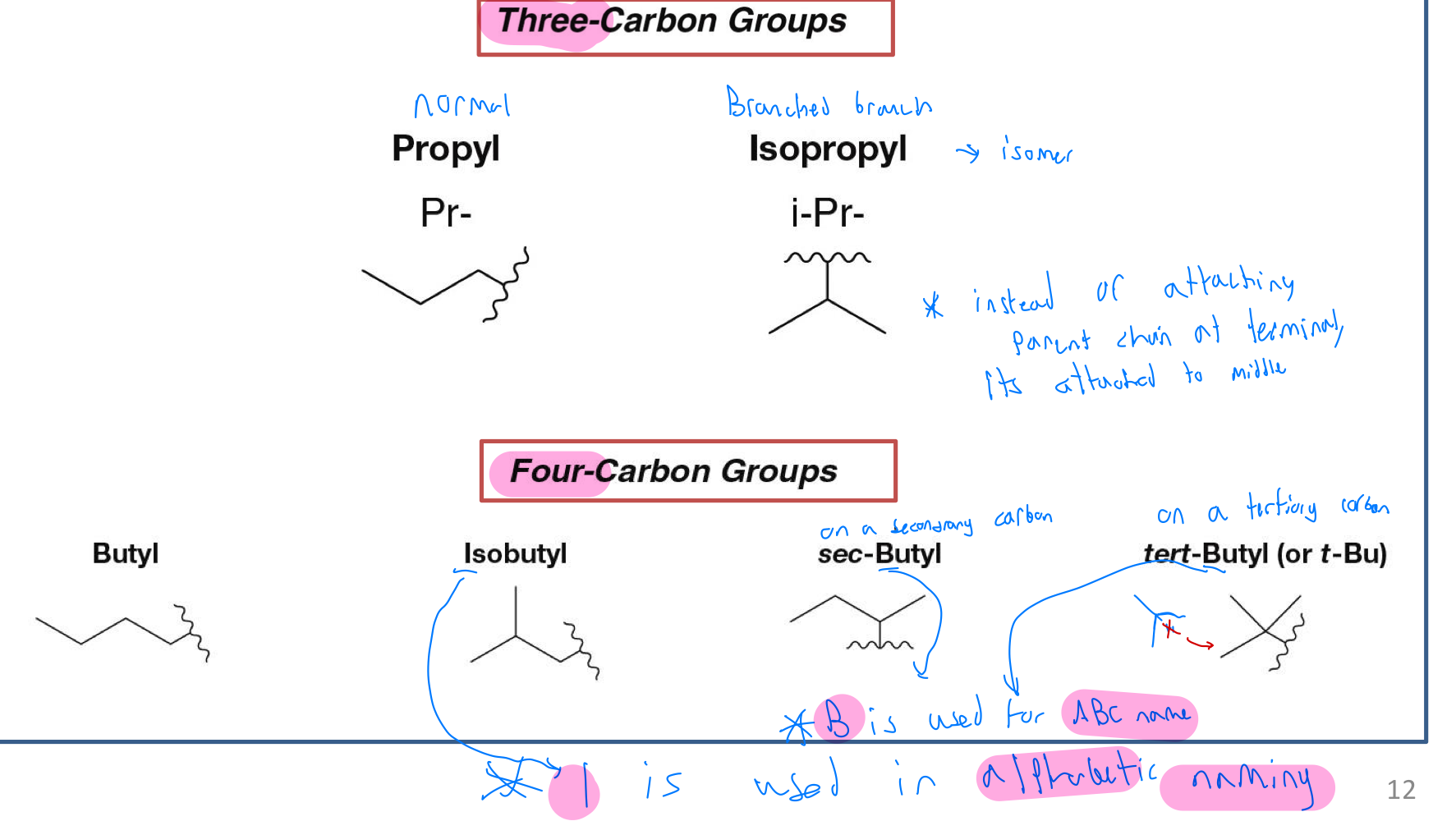
naming branched branches (three and four carbon groups)
three carbon groups: Parent chain attached to middle of substituent chain instead of end like usual. Add iso to the front: ex: isopropyl from propyl
four carbon group:
attached to one of three star branches: isobutyl
Attached to the middle of a chain: sec-butyl
Attached to the star branches but this ttime in the middle: tert-butyl
consider their alphabetic order
naming single ring cycloalkanes unbranched
add cyclo to the name of the parent chain
ex: cyclopropane
single branched cycloalkane
no number is placed before branch
e.g: tert-butylcyclohexane
double branched cycloalkane
number substituent first in ABCs with position 1, then go around in a direction that gives the second branch the lowest number.
Naming triple branched cycloalkanes
find all the possible combinations of numbering the branches, then choose the one that gives the lowest overall numbers
Then list then in abc order when naming it
When a ring is the substituent
Position - cycloxxxxyyyane. Ex: 1 - cyclobutylpentane. If there are two rings, then do dicyclo…
Naming alkylhalides (2 methods)
1: haloalkanes group treated like substituents: chloro, bromo, iodo, fluoro. Ex: 1-fluorohexane
2: or name the alkyl group and add halide. Ex: propyl fluoride
Naming alcohols
name like an branched alkane but
Parent chain is longest chain with OH attached to a carbon
Give the carbon with the oh group the lowest number
Change the ending of the name from e to -position-ol: ex: 4- methylhexane to 4-methylhexan-1-ol
If there is more than one OH, call it diol, triol,
If there is a ring, name the carbon with oh, 1. And then give lowest numbers to others
nomenclature of alkenes
named same as alkanes but with en infix not an.
select longest chain containing c=c
give c=c lowest position unless there is an alcohol, give it to the c on oh
if there is a cis or trans isomer, add that to front of name
if more than one double bond use diene, triene, etc
naming cycloalkenes
give the c=c 1 and 2 positions, so that the substituent has the lowest number as well. unless it has an alcohol
dont add cis
naming alkynes
same as alkenes but with yn infix.
oh group lowest number
if double and triple, give lowest set of locants overall, if equivalent, give lowest to double bond.
en is written before yn as infix
terminal alkyne
when sp c is attached to h