Exam 1 study guide
1/368
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
369 Terms
What is a microorganism? What are some examples?
Living organisms that are too small to be seen w/out magnification
examples: Bacteria, archaea, protozoa, fungi, helminths, viruses, algae
Bacteria characteristics
prokaryotic
peptidoglycan cell wall
unicellular
asexual (by binary fission)
circular DNA
some autotrophic (photosynthesis) & some heterotrophic
Archaea characteristics
prokaryotic
pseudomurein cell wall
unicellular
asexual (binary fission)
thermophiles (heat lovers)
halophiles (salt lovers)
methanogens (produce methane as a waste product of respiration)
autotrophic and heterotrophic
circular DNA
Fungi characteristics
eukaryotic
chitin cell wall
heterotrophic
unicellular (yeast) or multicellular (molds and mushrooms)
sexual or asexual
linear DNA
protozoa characteristics
eukaryotic
usually lacks cell wall
heterotrophic
unicellular
sexual or asexual
moves by: pseudopods (engulfs it), flagella (tall and long legs), cilia, some are non-mobile (not able to move)
Algae characteristics
eukaryotic
cellulose cell wall
autotrophic (photosynthesis)
unicellular or multicellular
sexual or asexual
often contain pigments like green, red, or brown
virus characteristics
acellular (needs a host cell, not made of cells)
obligate intracellular parasites
DNA or RNA
may be enveloped or naked
has a capsid protein coat
neither autotrophic/heterotrophic
helminths characteristics
no cell wall
heterotrophic
sexual or asexual
have microscopic stages (can eventually become large and carry diseases)
What makes viruses different from other microorganisms? for example, are viruses living and what is the structure of viruses
viruses are acellular and need a host cell in order to reproduce. They may be enveloped or naked. They have a capsid or a protein coat. They can either have DNA or RNA
What are helminths
flat worms and round worms (animal cells)
what does it mean to say that a microbe is ubiquitous
they are found everywhere on earth such as deep in earth’s crust, inside the bodies of plants and animals. Essential to life
genetic engineering
manipulates the genetic of microbes, plants, animals for the purpose of creating new products and GMOs
Recombinant DNA tech
makes it possible to transfer genetic material from one organism to another and deliberately alter DNA
bioremediation
uses microbes already present or introduced internationally to restore stability or clean up toxic pollutants
The term used to describe a disease-causing microorganism is
pathogen
What is the leading cause of infectious death in the US? what is the leading cause of infectious death in the world?
In the US< influenza and pneumonia. In the world, low respiratory system
taxonomy
the science of classifying living things
nomenclature
the assignment of scientific names to the various taxonomic categories and to individual organisms
classification
the orderly arrangement of organisms into a hierarchy
identification
the process of discovering and recording traits of organisms so that they can be placed in an overall taxonomic scheme
who developed the formal system for classifying and naming organisms
carolus linnaeus
each organism has 2 names. the first is the (1) and the second name is the (2)
genus
specific epithet/species
How are the names of organisms written
The genus is the first and the first letter is capitalized. The species is second and is lower cased. Both names are underlines (separately) or italicized (separately and if typed)
Carl Woese proposed that organisms may be classified into of one of three different domains by analysis of their
rRNA analysis
What are the three domains proposed by Carl Woese
Bacteria, archaea, and eukarya
How are organisms classified and organized into several descending ranks
domain, kingdom, phylum/division, class, order, family, genus, species
“Do Keep Pots Clean Or Family Gets Sick
who was the first to report that living things were composed of little boxes or “cells” from looking at the cork
Robert Hooke - invented the cell theory
cell theory
all living rhings are composed of cells and come from pre-existing cells
Who was the first to look at living microorganisms with a microscope
Anton van Leeuwenhoek (analyzed water and saw living things
Redi experiment
sealed jar with decaying meat → no maggots; open jar w/out decaying meat → maggots
supports biogenesis (life came form life)
Needham experiment
broiled broth and then covered it w/ a cap → bacterial growth
supports spontaneous generation (life came from nothing)
Spallanzan experiment
covered broth and boiled it → no growth
supports biogenesis, but fans say that boiling destroyed vital force
Pasteur experiment
broth placed in s-shaped flask, heated, not sealed → no growth due to day and microorganisms trapped in bend
supports biogenesis
pasteurization
application of gentle heat for a short amound of time
What are Koch’s postulates for? what are the steps for koch’s postulates
to determine whether or not an organism is pathogenic and which disease it caused
the same pathogen must be present in every case of the disease
the pathogen must be isolated from the diseased host and grown in pure culture
the pathogen from the pure culture must cause the disease when it is inoculated into a healthy, susceptible laboratory animal
the pathogen must be isolated from the inoculated animal and msut be shown to be the original organism
How did jenner produce the first vaccine
he found that milk maids were immune to smallpox due to being exposed to cowpox and cows. He would explore individuals to material from cowpox lesion (similar to smallpox). Then exposed them to smallpox and there’s no reaction
immunity
protection from disease provided by vaccination
what type of enzymes were discovered in the 1970s that is used to cut DNA in specific ways
restriction enzymes - molecular “scissors” inside bacteria → allow recombinant/genetic engineering and treatment for diseases
What technique was invented in the 1980s by Kary Mullis, that was awarded the Nobel Prize in 1993, that is used to amplify and subsequently analyze DNA
Polymerase Chain Reaction (PCR) - detect tiny amounts of DNA and make many copies of DNA. Method for discovering new organisms, diagnosing infectious diseases, and for forensic work
prokaryotic
has no nucleus
peptidoglycan
protein sugar wall
chitin
a fibrous substance consisting of polysaccharides and forming the major constituent in the exoskeleton of arthropods and the cell walls of fungi.
cellulose
an insoluble substance which is the main constituent of plant cell walls and of vegetable fibers such as cotton. It is a polysaccharide consisting of chains of glucose monomers.
photosynthesis
light-fueled conversion of carbon dioxide to organic material
accompanied by the formation of oxygen
anoxygenic photosynthesis
occurred in bacteria before plants evolved
did not produce oxygen as a byproduct
more efficient in extracting energy from sunlight
oxygenic photosynthesis
evolved from anoxygenic photosynthesis
photosynthetic microorganisms are responsible for 70% of the earth’s photosynthesis
spontaneous generation
the hypothesis that living organisms arise from nonliving matter; a “vital force” forms life
biogenesis
the hypothesis that the living organisms arise from preexisting life
aseptic technique
techniques that prevent contamination by unwanted microorganisms. Invented by Louis Pasteur
Germ theory of disease
proved that bacteria cause disease
Koch
proved that microorganisms can cause disease
Lister
First to use disinfectants in surgical procedures
fleming
discovered penicillin (an antibiotic)
Semmelweis
advocated handwashing between patients
Human microbiome project (HMP)
studied the diverse microbial communities that live in and on the human body, and their roles in human health and disease
What is the term for anything that has mass and takes up space
matter; fundamental unit of matter is the atom
know the parts of the atom. Name which parts are positively charged, negatively charged, and neutral. Which parts are in the nucleus. Which part of the atom is involved in chemical bonding
protons: + charge; in the nucleus
neutral: neutral charge; in the nucleus
electrons: - charge; involved in chemical bonding
What are elements? What defines an element?
Any substance that cannot be reduced to any simpler set of constituent substances. Defined by # of protons in its nucleus
Fluorine has 9 protons, 9 neutrons, and 9 electrons. What is fluorine’s atomic number and mass number
atomic number: 9 = # of protons
mass number: 9 + 9 = 18 → # of protons + neutrons in the nucleus
what are isotopes? what is it about carbon-12, carbon-13, and carbon-14 that makes makes them all carbon? what is different between carbon-14 and carbon-12
same # of protons and electrons; different neutrons
c-14 = 8 neutrons (unstable/radioactive isotope that gives off energy)
c-12 = 6 neutrons
How many electrons in the first, second, and third electron shell. When is an atom stable
first = 2 electrons
second = 8 electrons
third = 8 electrons
an atom is stable when all valence electrons are filled. lowest energy state. atoms can share, donate, or recieve electrons
what are ions? How do you know if an atom is an ion
an atom or molecule with an electrical charge resulting from gain/loss of electrons
when an electron is lost, a positive charge (cation) results
when on is gained, a negative charge (anion) results
covalent bond
atoms share one or more electrons
ionic bond
atoms lose and accept electrons from each other
hydrogen bond
a covalently lined hydrogen atom can react with an electronegative atom (like oxygen)
compound
a substance consisting of 2 or more different elements combined in a fixed ratio
molecule
a group of atoms bonded together, representing the smallest fundamental unit of a chemical compound that can take part in a chemical reaction.
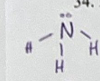
Is NH3 molecule polar or nonpolar
polar b/c N has an unequal sharing of electrons (partial charges are created)
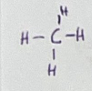
Is CH4 molecule polar or nonpolar
nonpolar b/c C has equal sharing of electrons (no partial charge)
Why does sodium and chloride form a bond?
Sodium has 11 protons and 11 electrons and chlorine has 17 protons and 17 electrons. So when they form an ionic bond, chlorine steals one electron from sodium to bond w/ one of their electrons, so, sodium loses 1 electron and becomes a cation and chlorine gains 1 electron and becomes an anion
What atoms do organic molecules always contain? What atom do inorganic molecules usually lack?
Carbon and hydrogen are always contained in organic molecules. Inorganic molecules usually lack hydrogen (and even carbon)
Why is water considered to be polar? in hydrogen bonding, what will the hydrogen of one water molecule bond with
It is polar because of difference in electronegativity. In hydrogen bonding, hydrogen bond between water molecules
What property of water allows water to be transported up a plant from the roots to the leaves
Adhesion allows water to defy gravity (water sticks together)
Water has a great capacity to absorb and retain heat. b/c of this, we say that water has a high
specific heat
specific heat
amount of energy required to raise the temperature of a substance by 1 C
What property of water allows life to live in water even when water freezes in colder climate
(ice is less dense than water) → water expands when frozen; h bonds are constantly forming and breaking; crystalline structures of ice holds the water in a ridged, less dense structure
solution
a completely homogenous mixture made of 2 or more substances
solute
substance is dissolved
solvent
he substance in which something is dissolved
when you add salt to water and the salt dissolves, what is the salt called
solute
what makes a compound hydrophobic
substances that repel water; substances can’t h-bond with water and nonpolar and non-ionic (vegetable oil)
What makes a compound hydrophilic
substances that h-bond with water; ionic compounds: salt; polar molecules: sucrose
acid
substances that release H+ in solution and increase H+ concentration
ex: lemon juice
base
substances that release OH- in solution and decrease H+ concentration
ex: household bleach
If an acid is added to water, will the pH of the solution increase or decrease? Why?
It will decease because the solution will have more H+
If a base is added to water, will the ph of the solution increase or decrease? Also, what happens to the hydroxide ion concentration as a result of a base being added?
It will increase because the solution will have less H+
What is a buffer and how does it work (ie. what happens when H+ is depleted or in excess)
a solution that resists change in pH
If H+ is depleted, buffers donate H+
If H+ is in excess, buffers accept H+
Chemistry
study of interactions between atoms and molecules
trace elements
life requires 25 essential elements such as iron, oxygen, carbon, hydrogen, nitrogen, etc.
outer shell is called
valence shell
outer electrons are called
valence electrons
chemical bonds
attractions between atoms
single covalent bond
the sharing of one pair of valence electrons
double covalent bond
the sharing of two pairs of valence electrons
highly electronegative atoms
have a high attraction for electrons
outer shells are nearly full
oxygen, nitrogen, and chlorine are very electronegative
Atoms with very little electronegativity
have a very low attraction for electrons
outer shells are nearly empty
hydrogen, carbon, and sodium are not very electronegative
ionic bond
when attraction holds te ions together. two ions w/ opposite charges attract each other
chemical reactions
making or breaking of bonds between atoms
Chemical energy
the potential energy stored within the chemical bonds of atoms and molecules. This change occurs during a chemical reaction