Cartilage Pathology
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
What is the Articular Epiphyseal Cartilage Complex and what does this become in the adult animal?
The Articular Epiphyseal Cartilage Complex (AECC) is the hyaline cartilage at the ends of long bones that serves both as the articular cartilage (which cushions the joint) and the epiphyseal growth cartilage (which contributes to bone growth in juvenile mammals).
It will become simply Articular Cartilage in the adult animal;
How is blood supplied to physeal growth plate?
• branches of the epiphyseal artery supply the resting zones of the growth plate
• branches of the metaphyseal artery form capillary loops at the metaphyseal side where endochondral ossification occurs
integrity of this vascular supply is critical to the process of endochondral ossification
What are the layers of the Articular Cartilage Complex (AECC)?
What supplies the blood needed for growth?
Structure found within the immature skeleton of a young, growing animal
articular cartilage (AC) overlies the temporary growth cartilage of the epiphysis epiphyseal cartilage (EC)
• highly dependent on a network of blood vessels (running in the "cartilage canals") from perichondrium & subchondral bone
• similar to physeal growth plate EC undergoes endochondral ossification
• contributes to growth/development of the epiphysis
How does articular cartilage appear? Where and when is it at its thickest?
→ Smooth, glistening & white to bluish colour
→ thickest in the young, and at sites of maximal weight-bearing
Describe the blood supply in articular cartilage?
Trick question
Adult articular cartilage
NO blood vessels, lymphatics or nerves
nutrients obtained by diffusion from synovial fluid
What cells compose the articular cartilage?
Chondrocytes (5% of the tissue)
95% is EXM (secreted by chondrocytes)
Which is composed of:
Proteoglycans
Aggrecan aggregate many proteoglycans
Collagen mainly type lI
Water 70-80% of matrix
Aggregates bind to hyaluronic acid which binds to water
Articular cartilage is a key component of ______ _____
synovial joints
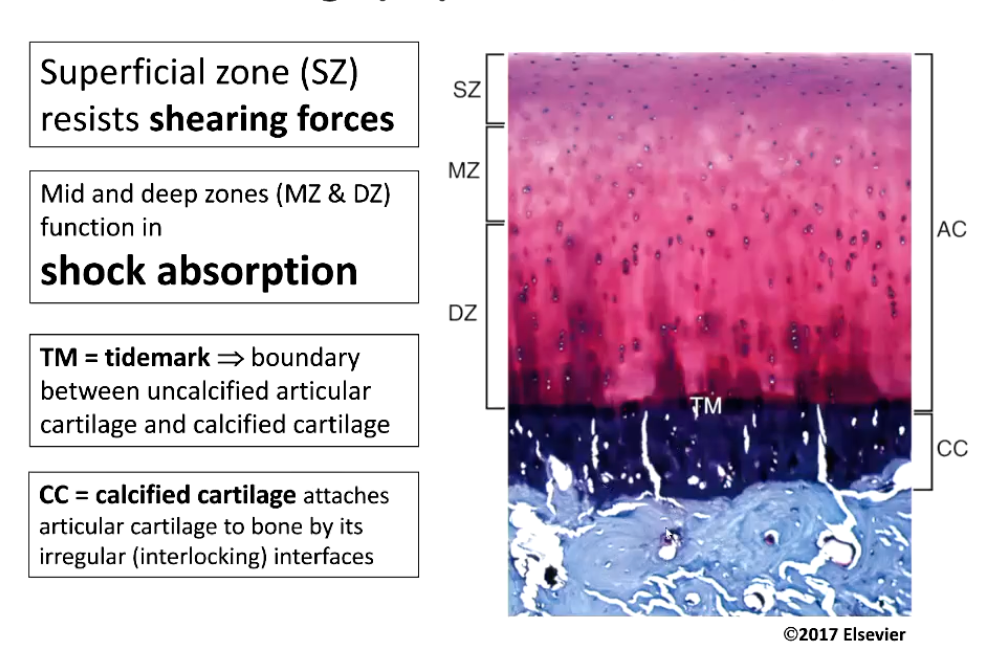
What are the different layers of articular cartilage and what function does each layer have?
Superficial zone (SZ) - resists shearing forces
Mid and deep zones (MZ & DZ) function in shock absorption
TM = tidemark → boundary between uncalcified articular cartilage and calcified cartilage (Attaches articular cartilage to underlying subchondral bone)
CC = calcified cartilage attaches articular cartilage to bone by its irregular (interlocking) interfaces
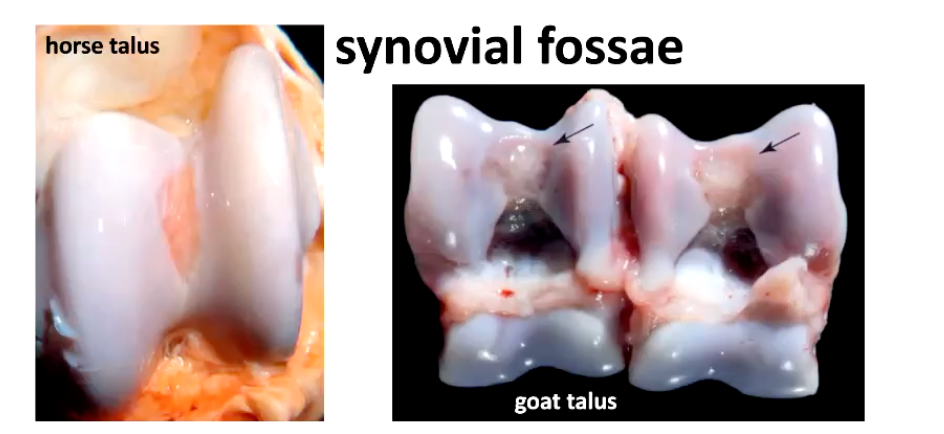
What are the synovial fossa?
What should they NOT be confused with?
• observed in some joints of ruminants, horses and pigs
Are non-articulating depressions near the midline of the joints
normal structures, bilateral and symmetrical acquired during joint modelling the first months of postnatal life
central depressions with distinct borders and a smooth, blue to pink surface, reflecting the proximity of the subchondral capillary bed
SHOULD NOT mistake synovial fossae for lesions in the articular cartilage or as indicators of collapsed subchondral bone
Describe how cartilage responds to injury.
1. Initial Injury
Haemorrhage → haematoma formation at the site of cartilage damage.
Platelets release PDGF and TGF-β → trigger inflammation and mesenchymal cell proliferation.
2. Early Repair Response
Chondrocytes near the lesion start producing matrix rich in proteoglycans and type II collagen.
Defects are usually filled with fibrocartilage, which attaches to adjacent hyaline cartilage.
In other organs, fibrous scar tissue forms similarly, but inadequate performance when subjected to mechanical loading.
3. Matrix Remodeling & Degradation
Aggrecanases → degrade aggrecan (proteoglycan component).
Matrix metalloproteinases (MMPs) → degrade collagen and other matrix proteins.
Normally inactive, but can be activated by degenerating chondrocytes or inflammatory cells.
Tissue inhibitors of metalloproteinases (TIMPs) → balance MMP activity to limit damage.
4. Consequences for Articular Cartilage
Loss of proteoglycans → alters cartilage hydration and hydraulic permeability.
Progressive loss of matrix → changes biomechanical properties.
Fibrocartilage repair tissue is mechanically weaker than native hyaline cartilage.
Deep injuries often lead to new bone formation at the base to restore the subchondral plate.
Why do MMPs and aggrecanases become active when cartilage becomes injured?
1. Normally
Aggrecanases and MMPs are present in the matrix in inactive forms.
This prevents unnecessary breakdown of cartilage.
2. Activation triggers
a) Degenerating or reactive chondrocytes
Injured chondrocytes release signals (cytokines, ROS, nitric oxide) that activate latent enzymes.
b) Inflammatory cells
Macrophages, neutrophils, and synovial cells secrete pro-inflammatory cytokines like IL-1 and TNF-α.
These cytokines activate MMPs and aggrecanases to degrade damaged matrix.
3. Purpose (in context)
Helps remove damaged matrix so new tissue can form.
But excessive activation leads to progressive cartilage breakdown, as seen in osteoarthritis or severe injury.
Why is there enzyme-mediated cartilage matrix degeneration in the case of articular cartilage injury?
Cartilage injury leads to inflammation and chondrocyte activation, which upregulates proteolytic enzymes (MMPs, aggrecanases). These enzymes degrade the extracellular matrix, causing progressive loss of cartilage structure and function.
What does the loss of proteoglycan in articular cartilage injury cause?
loss of proteoglycans = altered hydraulic permeability of cartilage
Leads to progressive loss of cartilage matrix
Alteration of cartilage biochemical and biomechanical processes
What is chondrodysplasia?
A hereditary developmental cartilage disorder, with primary lesions affecting cartilage growth and subsequently skeletal development
Clinical manifestation = disproportionate dwarfism
Chondrodysplasia should be distinct from?
• chondrodystrophy induced by Manganese deficiency
• metabolic bone defects ("rickets")
skeletal abnormalities in animals with lysosomal storage disorders (Mucopolysaccharidosis, Gangliosidosis)
What are the features of lethal bulldog type bovine chondrodysplasia?
Affecting miniature cattle: (m Jersey, m Scottish Highland, m Belted Galloway)
• extremely short limbs usually rotated
• short domed head with protruding mandible
• short vertebral column
• cleft palate
• large ventral abdominal hernia
These features are not compatible with life, calves are aborted.
What are the causes lethal bulldog type bovine chondrodysplasia?
Cause: mutations in Aggrecan (ACAN) gene
What are the features of spider lamb syndrome?
Affects suffolk and hampshire sheep breeds, caused by Autosomal recessive with incomplete penetrance
• disproportionately long limbs and neck
• shallow body
• scoliosis and/or kyphosis of the thoracic spine
• concave sternum & other sternal deformities
• valgus deformity of forelimbs below the carpus = knock-kneed appearance
Cause - point mutation in FGFR3 gene (fibroblast growth factor receptor 3), compromises the formation of cartilage, disorganized ossification centers
What are the features of texel sheep chondrodysplasia?
autosomal recessive trait with variable expression
reduced growth rate
shortened neck and legs
varus forelimb deformities and a wide-based stance
severely affected lambs
progressive reluctance to walk
often die within 4 months
both articular & physeal cartilage
disorganization of chondrocytes
foci of chondrolysis - a process of loss of chondrocytes & matrix
coalescing into clefts / cystic spaces
Cause
1-bp deletion in SLC13A1 gene Sodium-sulphate co-transporter
What are the features of chondrodysplasia in the Alaskan Malamute?
autosomal recessive, leads to disproportionate, short-legged dwarfism
haemolytic anaemia (possibly an incompletely dominant trait associated with the chondrodysplasia)
Rx changes apparent as early as 7-10 days of age & more pronounced after 3 weeks
progressive deterioration during growth
bowing of radius and ulna
lateral deviation and enlargement of the carpus
sclerotic and abnormally shaped metaphyses
→ disruption of the metaphyseal blood supply
→ thus causes impaired vascular invasion of the developing growth plate and endochondral ossification process, we see
irregular thickening of growth plates in the limb bones
islands of hypertrophic cartilage extend into the metaphysis close to healing trabecular microfractures
Growth plate lesions are similar to rickets
What does the growth plate of a puppy with chrondrodysplasia look like?
irregular thickening of the physeal growth plates - segmental thickening of the hypertrophic zone in physeal growth plate
Primary spongiosa shows disrupted trabecular architecture, disorganized osteod spicules with microfractures
What are the features of feline physeal dysplasia & slipped capital femoral epiphysis?
most common in male and overweight cats
affected females often have affected male littermates
Siamese & Maine Coon over-represented, also common in DSH
Affected cats are typically young (2-4 years)
but older than the age of expected physeal closure (7-9 months)
We see:
persistence of dysplastic growth plates with disorganised chondrocyte clusters surrounded by abundant matrix, results in a fracture
changes also present in other physeal growth plate, but only the proximal femoral physis fractures
presumably because of the shear forces at that site
What is osteochondrosis?
Degenerative process affecting cartilage with a failure of endochondral ossification affecting:
articular-epiphyseal cartilage complex (AECC) & physeal / growth plate
Typically affects: young growing animals
• Pigs
• Horses
• Dog (large/giant breeds)
• Ruminants
(with anatomical predilection sites for lesion development)
What is the aetiopathogenesis of osteochondrosis?
Multifactorial
• Genetics
influence on body conformation → contribution to lesion development
• Trauma
promotes progression of earliest lesions to become more advanced/severe many young pigs have early lesions but most resolve in pigs that get more exercise, lesions are more likely to progress to advanced lesions
• Vascular factors
focal failure of vascular supply to growing cartilage
→ localised ischaemic necrosis
→ initiation of early subclinical lesions
What are the 3 forms or stages of Osteochondrosis?
Osteochondrosis latens
focal ischemic necrosis of growth cartilage, but not articular cartilage of the AECC (subclinical, may resolve without progression)
Osteochondrosis manifesta
retention of necrotic cartilage → failure of endochondral ossification grossly or radiographically visible focus of cartilage necrosis in subchondral bone (may or may not show clinical signs, can resolve by gradual removal of the necrotic cartilage focus)
Osteochondrosis dissecans (OCD)
necrosis dissecting through articular cartilage with cleft formation often resulting in flaps of articular cartilage (clinically relevant lesion, irreversible)
In Osteochrondrosis, what are the predilection sites for the following species?
Pig
Horse
Dog
Cattle
ALL sites of high dynamic load during movement / exercise
Pigs
• distal femur (especially the medial femoral condyle)
• humerus (condyles and head)
Horse
• distal femur (medial condyle and both trochlear ridges)
• distal tibia (cranial intermediate ridge and medial malleolus)
• talus (trochlear ridges)
• articular processes of the cervical vertebrae
Dog
• humerus (head and medial condyle)
• distal femur (both condyles)
• talus (medial and lateral trochlear ridges)
Cattle
• talus (medial and lateral trochlear ridges)
• distal femur (both trochlear ridges)
What type of dogs are typically affected by Osteochondrosis dissecans?
• Dog, head of humerus
• Usually young males of large & giant breeds
• Cartilage flap (C)
Where are the cartilage cannals most vulnerable to failure in the foal?
Distal femur, process of transversing tissue junctions renders the canal vessels vulnerable to failure, leading to OCD and subchondral bone cysts
Why can horses with septic arthritis and osteomyelitis (Mimicking osteochondrosis-like lesions) still be used for breeding?
Sepsis in the cartilage canals of foals can cause necrosis, neutrophil infiltration, and granulation tissue formation that lead to ischemic chondronecrosis and delayed ossification within the articular-epiphyseal cartilage complex or growth plate. These infection-induced changes can closely mimic the lesions of osteochondrosis (OC/OCD) on imaging and histopathology, but they arise from bacterial colonization and inflammatory vascular damage rather than genetic or developmental failure of endochondral ossification. Because the pathogenesis is septic and not hereditary, horses that develop these OC-like lesions secondary to sepsis are not considered to carry or transmit osteochondrosis-predisposing genes. Therefore, horses with proven sepsis-related OCD lesions may safely be used for breeding without increasing the prevalence of genetically driven OC in the population.
How is osteochondrosis in physeal growth plate cartilage distinct from articular epiphyseal cartilage (AECC)?
Early lesions in physeal growth plate are:
cone-shaped foci of retained cartilage extending into the metaphysis
NO necrotic cartilage compared to AECC
Possible pathogenesis:
trabecular microfractures in primary spongiosa
→ interference with vascular invasion of mineralized cartilage during endochondral ossification
→ persistence of hypertrophic zone chondrocytes (retained wedges of hypertrophic cartilage in the metaphysis)
What are some possible proliferative lesions of cartilage?
Tumours and tumour-like lesions
• Chondroma
• Chondrosarcoma
• Osteochondroma
• Feline Osteochondromatosis
What are the main features of chondromas?
Benign tumour of cartilage
Rare in all species, most commonly reported in aged dogs & sheep
Slow growth rate, with deformation of affected bones
Generally painless swelling
Phalanges, cervical spine, ribs or other bones
What are the main features of chondrosarcomas?
How does it present clinically?
Malignant tumour of cartilage
More frequent in dogs (10% of primary bone tumours) mostly medium/large breeds, also reported in cats, uncommon in other species more common in flat bones, pelvis, ribs, nasal turbinates (long bones also possible). Presents with a slow growth but with local invasion, possible recurrence following surgery metastatic spread less frequent and with longer latency compared with OSA
Nasal chondrosarcoma = sneezing and epistaxis
Pelvic chondrosarcoma = masses possibly associated with constipation, tenesmus, hind limb lameness
Long bones chondrosarcoma = painful swelling, pathological fractures
Extraskeletal chondrosarcomas rare but possible: Heart, arteries, retroperitoneum, bladder, omentum, lung
What is osteochondroma?
What species are affected?
What are the clinical signs?
cartilage-capped osseous outgrowth arising from the surface of a bone formed by endochondral ossification
monostotic (single bone affected) = solitary osteochondroma
polyostotic (multiple bones affected) = osteochondromatosis
dogs & horses
osteochondromas typically in young animals during active bone growth
Clinical signs depend on size and location as benign disfigurement, lameness, pain, paresis and paralysis
→ typically enlarge in synchrony with physeal growth
→ growth ceases once the skeleton reaches maturity
→ continued growth after skeletal maturity suggests
malignant transformation
What is feline osteochondromatosis?
Feline osteochondromatosis shows progressive growth after reaching skeletal maturity
(osteochondromas in dogs and horses are developmental)
prognosis poor
typically affecting multiple bones (skull, ribs, vertebrae, other...)
oncogenic retroviruses (FeLV/FeSV) detected in cases of osteochondromatosis
speculated theory of viral-induced proliferation in the lesions
What are the differences between primary and secondary synovial chondromatosis?
Primary (pathogenesis unknown)
Idiopathic nodular cartilaginous metaplasia of the synovium mostly dogs (large & giant purebreds) other species rarely affected
typical presentation: lameness & painful swelling of a single joint
cartilage nodules NOT visible with x-rays
can be detected with echo, CT and contrast arthrography
Secondary
Associated with chronic degenerative joint disease (DJD)
nodules less numerous (compared with primary SC) and often mixed with other forms of synovial proliferation and metaplasia
erosive changes in the articular cartilage are more marked than the synovial proliferation
What are the possible mechanisms behind secondary synovial chondromatosis?
pieces of articular cartilage that break free
metaplasia of the hyperplastic synovium into cartilage, which
undergoes ossification to form bony nodules (osteophyte formation)
These lesions can detach and become free floating within the synovial fluid (joint tophi).
How does the articular cartilage respond to injury in the case of a full thickness injury of articular cartilage, including the subchondral bone?