Hw 4: Aromatic Compounds
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
29 Terms
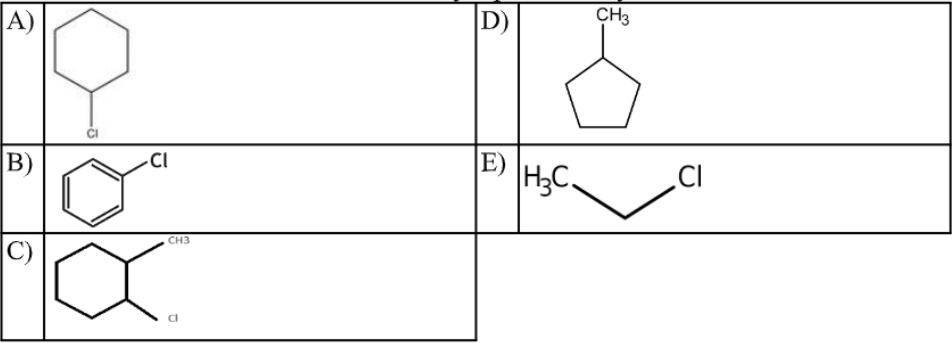
The structure of chlorobenzene is correctly represented by:
B

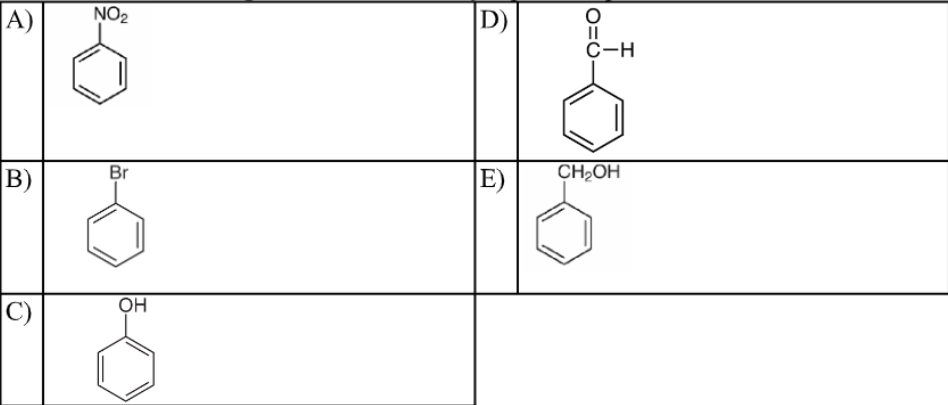
Which of the following structures accurately represents phenol?
C


The name of the following molecule is:
A) toluene
B) ethylbenzene
C) cumene
D) styrene
E) anisole
Anisole
Which dibromobenzene can form only one tribromobenzene?
A) o-dibromobenzene
B) m-dibromobenzene
C) p-dibromobenzene
D) cumene
E) styrene
p-dibromobenzene
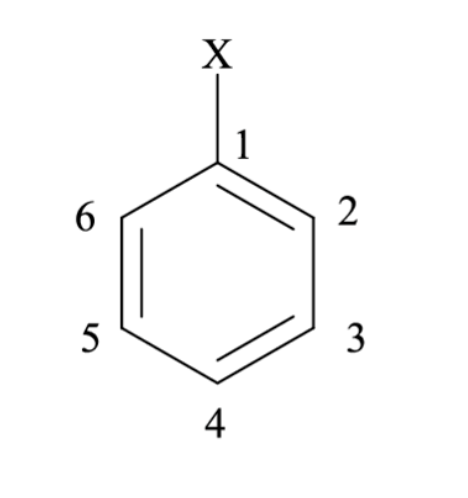
Using the following monosubstituted benzene, which position would be meta to X?
A) 1,4
B) 2,6
C) 3,5
D) 2,3
E) 5,6
3,5
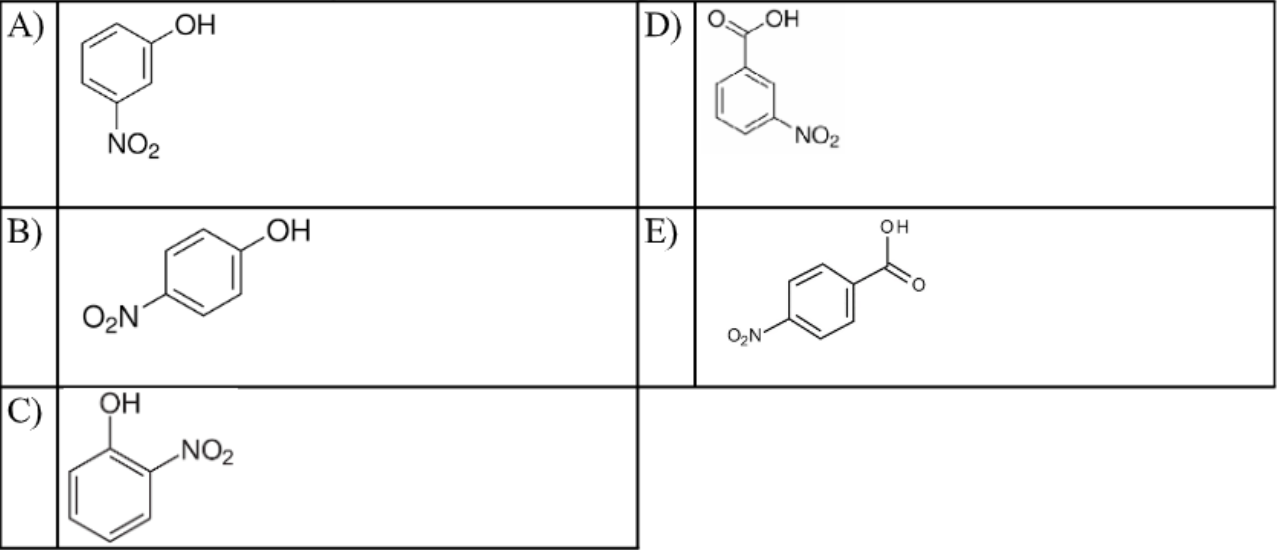
Which of the following molecules is o-nitrophenol?
C

What is the name of the following molecule?
PhCH2CH2CH=CH2
A) styrene
B) 4-phenyl-1-butene
C) 1-phenyl-3-butene
D) 3-benzyl-1-propene
E) allylbenzene
4-phenyl-1-butene
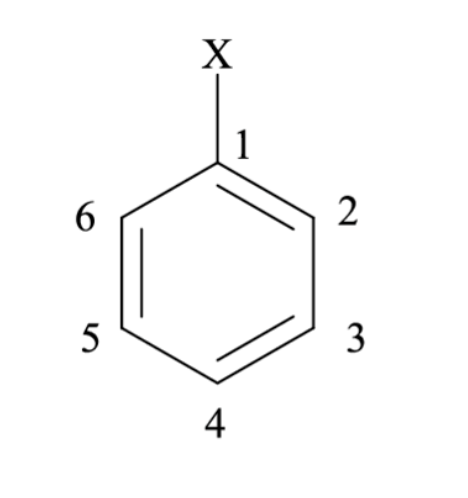
Which position would be para to X?
A) 1
B) 2
C) 3
D) 4
E) 6
4
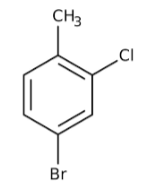
The correct name for
A) 2-chloro-4-bromotoluene.
B) o-chlorobromotoluene.
C) 1-bromo-3-chloro-4-methylbenzene.
D) 4-bromo-2-chlorotoluene.
E) p-chlorobromotoluene.
4-bromo-2-chlorotoluene.
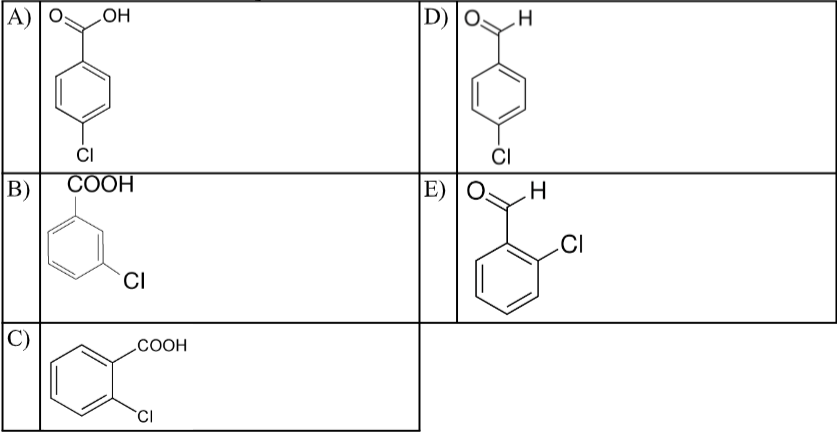
The structural formula for p-chlorobenzoic acid is:
A

Which of the following statements about benzene is FALSE?
A) the molecule is planar and each carbon is at a corner of regular hexagon
B) there are two resonance structures of equivalent energy
C) the bond angles at each carbon center is equal, 120º
D) the typical mechanism by which reactions occur is by addition
E) The bond lengths between each two carbons of the rings are equal
The typical mechanism by which reactions occur is by addition
Which statement about benzene is true?
A) All six hydrogens in benzene are chemically equivalent.
B) Benzene decolorizes bromine solutions.
C) The molecule is planar, and each carbon is at the corner of a regular hexagon.
D) Both a and c are true.
E) Both b and c are true.
Both a and c are true
Which of the following is NOT an electrophile in an electrophilic aromatic substitution
reaction?
A) NO2+
B) (CH3)3C +
C) SO3
D) Cl -
E) all are
Cl -
Which of the following groups is a nucleophile?
A) NO2+
B) (CH3)3C +
C) SO3
D) Cl -
E) all are
Cl -
Which heterocyclic compound is commonly found in DNA and RNA bases?
A) Pyridine
B) Furan
C) pyrol
D) Pyrimidine
E) Thiophene
Pyrimidine
Which heterocyclic compound is commonly found in vitamin B6?
A) Pyridine
B) Furan
C) pyrol
D) Pyrimidine
E) Thiophene
Pyridine
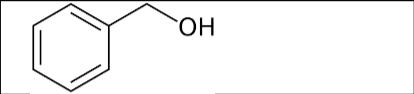
What is the name of the following compound?
Benzyl Alcohol
Nitrobenzene can be prepared by treating benzene with:
A) nitrous acid
B) nitric acid
C) Nitrogen molecule
D) Nitrate acid
E) nitrogen
Nitric Acid
What is a heterocyclic compound?
A) A compound containing only carbon and hydrogen in a ring
B) A compound with a ring that includes atoms other than carbon
C) A compound with an open-chain structure containing atoms other than carbon
D) A compound with only aromatic rings
E) A compound with only fused rings
A compound with a ring that includes atoms other than carbon
If p-nitrophenol is treated with chlorine in the presence of AlCl3 , the only trisubstituted product
observed is:
A) 2-chloro-4-nitrophenol
B) 3-chloro-4-nitrophenol
C) 3-chloro-5-nitrophenol
D) 4-chloro-2-nitrophenol
E) 4-chloro-3-nitrophenol
2-chloro-4-nitrophenol
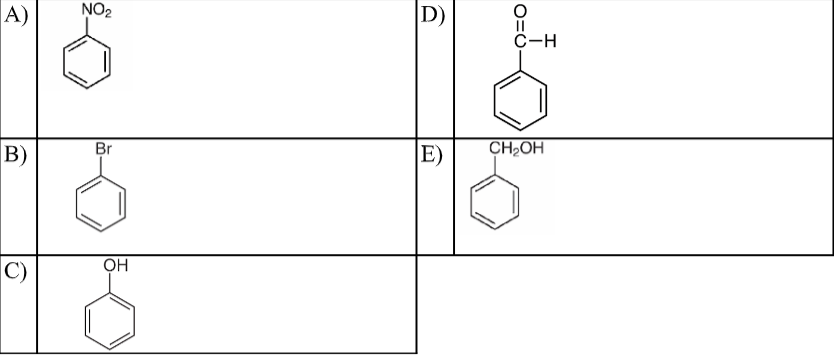
What is the structural formula for benzaldehyde?
D

The name of the product of the following reaction is:
Benzene + HNO3 in presence of sulfuric acid
A) benzenesulfonic acid
B) aniline
C) benzoic acid
D) nitrobenzene
E) anisole
Nitrobenzene
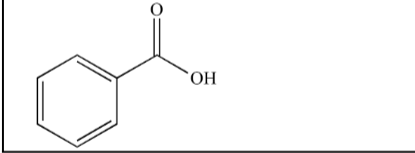
What is the name of the following compound?
A) aniline
B) anisole
C) benzoic acid
D) phenol
E) toluene
Benzoic acid
Why is benzene more stable than expected based on its structure?
A) It undergoes addition reactions
B) It contains alternating single and double bonds
C) It has localized π bonds
D) It has delocalized π electrons over the ring (aromaticity)
E) It undergoes substitution reactions
It has delocalized π electrons over the ring (aromaticity)
The polycyclic aromatic hydrocarbon benzo[a]pyrene is a known carcinogen found in soot and tobacco smoke. How many benzene rings are there in its structure?
A) 1
B) 2
C) 3
D) 4
E) 5
5
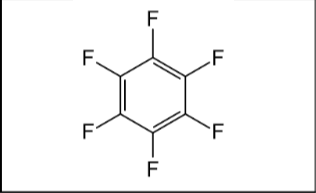
What is the name of the following compounds?
A) Benzene
B) fluorobenzene
C) hexafluorobenzene
D) o-fluorobenzene
E) p-fluorobenzene
Hexafluorobenzene
What type of product is formed in a Friedel–Crafts acylation of benzene?
A) carboxylic acid
B) An alcohol
C) A ketone
D) An aldehyde
E) HNO3
A ketone
What is the name of the following compound?
PhCH2Cl
A) Phenyl chloride
B) Benzyl chloride
C) benzoic acid
D) chlorobenzene
E) chloromethylphenol
Benzyl Chloride
Write the 3 criteria for aromaticity.
Be Cyclic
Be Planar
Have a continuous ring of p orbitals on each atom of the ring (Fully conjugated: alternate single and bond)