bonding (tania please study this)
5.0(2)
5.0(2)
Card Sorting
1/81
Earn XP
Description and Tags
wait i didnt know i could type a desc omg hello favs!! how have yall been?? tbh a lil worried for this one...
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
82 Terms
1
New cards
are atoms more stable with a full shell or a half full shell
full shell- the atom/ion has all the valence electrons it needs
2
New cards
what is formed when an atom gains or loses electrons
an ion
3
New cards
valence electrons (typically) are equal/related to the
group number
4
New cards
what is a lewis dot diagram
the element symbol surrounded by dots that represent valence electrons
5
New cards
the charge on the ion is known as the
oxidation number
6
New cards
how are ions written
element symbol w superscript showing the charge
7
New cards
ionic bond is formed when
electrons are transferred from one atom to another
8
New cards
metals __ electrons and nonmetals __ electrons
lose, gain
9
New cards
atoms are attracted to each other due to
opposite charges (one element has a net pos charge (the metal/cation) and the other has a net neg charge (the nonmetal/anion) - think of placement on the periodic table))
10
New cards
ionic substances are sometimes called
salts
11
New cards
salts are generally
neutrally charged (ex: NaCl)
12
New cards
to name an ionic compound, first name the ____ then the ____ and change the ending to _____
cation, anion, -ide
13
New cards
to write a chem formula:
first find the charges and determine how many of each ion you need to form a neutral compound
14
New cards
if the cation is a _____ ___, then you specify the charge with a ____ _____
transition metal, roman numeral
15
New cards
three transition metals where roman numerals are not needed
argon (1+), zinc (2+), and cadmium (2+) (these always have the same charges)
16
New cards
two group A metals that need roman numerals
sn (2+ or 4+), Pb (2+ or 4+) (these atoms have variable charges)
17
New cards
what is a polyatomic ion
groups of atoms that behave as one unit
18
New cards
when do polyatomic ions need parentheses
when more than one is used in a formula, otherwise they are treated like a single ion
19
New cards
what are the three types of bonds
ionic, covalent and metallic
20
New cards
how is an ionic bond formed?
electrons are transferred from a metal atom (left side of the staircase) to a nonmetal atom (right side of the staircase)
21
New cards
what happens to the metal and nonmetal when they transfer electrons? (ionic bond)
they form their respective ions
22
New cards
why are metals and nonmetals attracted to each other? (ionic bond)
cations and anions are attracted to each other
23
New cards
how do you name ionic bonds/ions
name the metal, then the nonmetal and change the ending of the nonmetal to -ide
24
New cards
what is the structure of an ionic bond
lattice (crystal) structure is present in many ionic compounds
25
New cards
what are properties of the ionic bond lattice structure?
good electrical conductor, high melting point, strong pos-neg attraction, water soluble
26
New cards
ex of a VERY COMMON ionic bonded molecule
NaCl (salt)
27
New cards
when/how is a covalent bond formed?
electrons between elements r shared (shared to fill each element's octet)
28
New cards
elements with covalent bonds are almost always
nonmetals!! (right of the staircase + hydrogen)
29
New cards
what are covalent compounds called
molecules
30
New cards
how do u name a covalent compound?
prefixes (will go in depth later) + the -ide ending
31
New cards
nonmetals bonded to each other have (similar/different) properties to regular nonmetals
similar - properties include low melting point, poor conductors, low solubility in water
32
New cards
ex of covalent compounds
water (h20), ammonia (NH3), chlorine (Cl2)
33
New cards
how is a metallic bond formed
when a metal is bonded to another metal (woah man who saw that coming!)
34
New cards
why are metallic bonds related to the term "sea of electrons"
electrons move within the metallic atoms
35
New cards
what does the movement of electrons in metallic bonds allow for
ease of electron transfer between atoms, atoms can slide past each other, lustrous, very high melting point
36
New cards
alloys
different metallic atoms bonded to one another exhibit unique properties due to difference in atomic size
37
New cards
what is it called when molecular compounds share their valence electrons?
a covalent bond!
38
New cards
what do covalent bonds form between?
two nonmetals
39
New cards
how do you name a covalent compound?
name the first element, then name the second one and change the ending to -ide (but use prefixes to show amount of atoms of each element)
40
New cards
mono
1 - omitted if on the first element
41
New cards
di
2
42
New cards
tri
3
43
New cards
tetra
4
44
New cards
penta
5
45
New cards
hexa
6
46
New cards
octa
8
47
New cards
hepta
7
48
New cards
nona
9
49
New cards
deca
i love deca tbh like its a 10/10 for me! (it means ten i hope yall thought thsi was funnt im really stressed tehse days) #gettheedge
50
New cards
what should you NEVER DO when writing covalent compound formulas
reduce subscripts (to clarify, never reduce subscripts)
51
New cards
can you use prefixes for ionic compounds
no!
52
New cards
what is a lewis dot diagram
a diagram that shows electrons available for bonding (the outermost valence electrons)
53
New cards
review the rules for how to draw a dot diagram
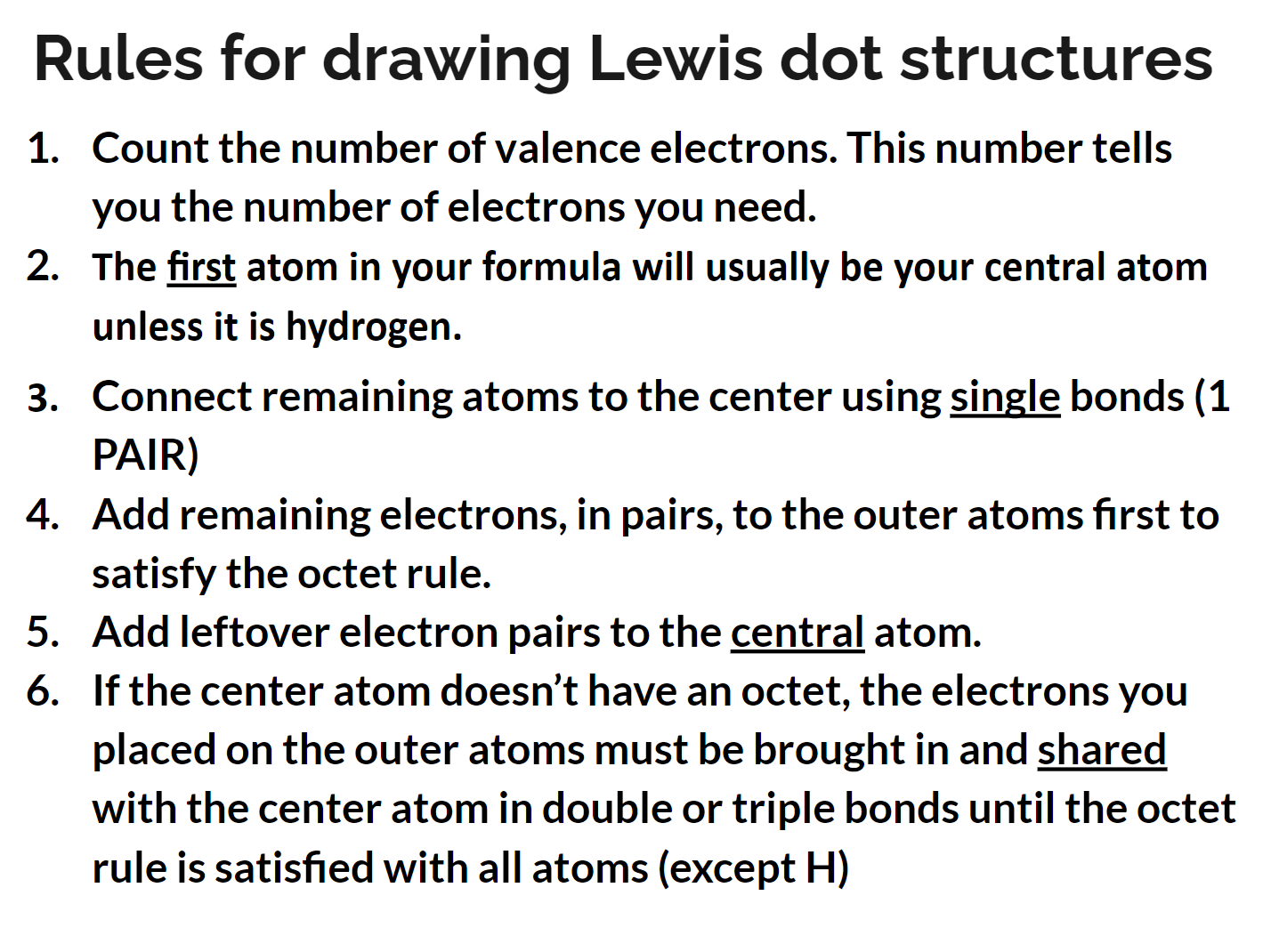
54
New cards
exception to the octet rule: hydrogen can only have __ valence electrons
2
55
New cards
exception to the octet rule: beryllium can only have __ electrons
4
56
New cards
exception to the octet rule: boron is stable with __ electrons
6
57
New cards
what periods have an expanded octet
any central atom that is from 3-7 have expanded octets
58
New cards
what is an expanded octet?
the ability (of a central atom) to accommodate more than 8 electrons
59
New cards
bonds, polarity: how large must the difference in electronegativity be for there to be an ionic bond formed?
greater than 1.7
60
New cards
what type of bond is formed when the difference in electronegativity is small?
a covalent bond
61
New cards
when is a covalent bond nonpolar?
electrons are shared equally and the overall charge is neutral (electronegativity difference is
62
New cards
when is a covalent bond polar?
when electrons are not shared equally (electronegativity difference between .4-1.7)
63
New cards
the more electronegative atom attracts more electrons
creating a partially negative region of the atom
64
New cards
the less electronegative region of the atom becomes
slightly positive
65
New cards
what are the two ways to show polarity of a molecule?
letter delta to show partial charges OR arrow that points in the more electronegative direction/atom
66
New cards
molecular polarity: what makes a molecule nonpolar?
if all the bonds are nonpolar
67
New cards
if a molecule has a ___ bond, then the whole molecule is polar
polar (this applies most of the time, but not always)
68
New cards
a molecule is nonpolar if
the central atom has no lone pairs + has all the same types of atoms attached to it (basically symmetry)
69
New cards
a molecule is polar if
the central atom has lone pairs OR the central atom has no lone pairs but different atoms attached to it
70
New cards
covalent bonds can be
single, double or triple
71
New cards
single bond (covalent)
two atoms share one pair of electrons (1 sigma bond)
72
New cards
double bond (covalent)
two atoms share two pair of electrons (1 sigma and 1 pi bond)
73
New cards
triple bond (covalent)
two atoms share three pair of electrons (1 sigma and 2 pi bonds)
74
New cards
hybridization
the concept of mixing atomic orbitals to form new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory
75
New cards
hybrid orbitals are formed by
mixing atomic orbitals of comparable energies
76
New cards
steps to determining hybridization

77
New cards
resonance
concept in which two or more Lewis structures for the same arrangement of atoms (resonance structures) are used to describe the bonding in a molecule or ion
78
New cards
how is resonance shown
double headed arrow is placed between the molecules resonance structures
79
New cards
what is a resonance structure?
various forms of the same molecule where the electrons have transferred from one region to another
80
New cards
what is a resonance hybrid
each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form.
81
New cards
formal charge equation
Formal charge = valence electrons - nonbonding electrons - ½ the bonding electrons
82
New cards
what is formal charge
Sometimes there are more than one possible structure so to find the most likely structure you will need to calculate the formal charge