4.5.1 Exothermic and Endothermic Reactions
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
What is a chemical reaction?
A transfer of energy to or from the surroundings, usually as heat
What type of reaction releases energy to the surroundings?
Exothermic
What type of reaction absorbs energy from the surroundings?
Endothermic
What happens to the temperature in an exothermic reaction?
The temperature increases as heat is given out
What are examples of exothermic reactions?
Combustion, respiration, neutralisation, hand warmers
What are examples of endothermic reactions?
Thermal decomposition, photosynthesis, citric acid + sodium hydrogen carbonate
What is the method for the temperature changes practical?
Measure the temperature change when two reactants are mixed in a polystyrene with lid (insulated)
What does the practical help identify?
If the reaction is exothermic or endothermic based on temperature change
What are sources of error for the practical?
Heat loss to air, inaccurate thermometer readings
How can the practical be improved?
Use a lid, insulation, digital thermometer
What are reaction profile diagrams?
Graphs that show how energy changes during a reaction
What does the Y axis on reaction profile diagrams show?
Energy
What does the X axis on reaction profile diagrams show?
Progress of reaction
What does an exothermic reaction profile look like?
Products are lower in energy than reactants
What does an endothermic reaction profile look like?
Products are higher in energy than reactants
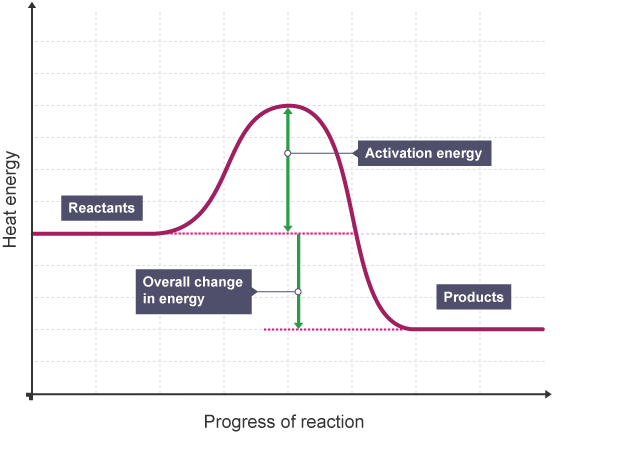
What reaction profile is this?
Exothermic
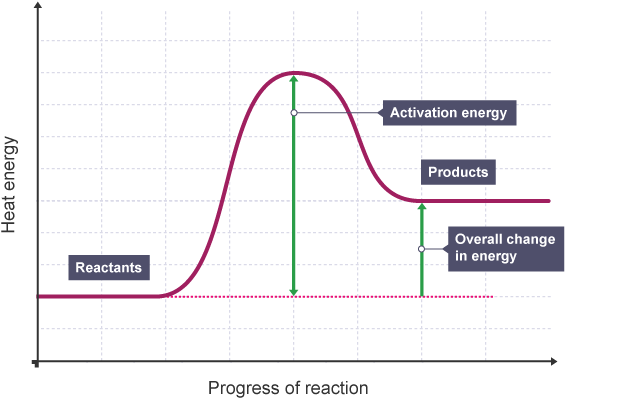
What reaction profile is this?
Endothermic
What happens when bonds are broken?
Energy is absorbed, which is an endothermic process
What happens when bonds are formed?
Energy is released, which is an exothermic process
What is the formula to calculate overall energy change in a reaction?
Energy change = Bonds broken - Bonds made
If the energy change is positive, what type of reaction is it?
Endothermic (more energy taken in than released)
If the energy change is negative, what type of reaction is it?
Exothermic (more energy released than taken in)
What is the method for calculating bond energies?
Multiply bond energy by number of bonds, add up total energy for bonds broken and formed, use energy change formula to find overall energy change
What is activation energy?
The minimum amount of energy needed to start the reaction
What lowers activation energy and how?
Catalysts, by providing an alternative pathway