[18] Equilibrium
1/37
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
What is the balanced chemical test for water? is this exothermic or endothermic?
CuSO₄ + 5H₂O → CuSO₄·5H₂O
This reaction is exothermic - heat is released when water binds to the salt.
What is Dynamic Equilibrium
Rate forwards equals rate backwards and the concentrations of reactants and products remain constant.
what is meant by equilibrium?
where the forward and reverse reactions occur at the same rate, so the concentrations of reactants and products remain constant.
What is meant by dynamic equilibrium in a reversible reaction?
The forward and backward reactions occur at the same rate.
What conditions are required for dynamic equilibrium to be established?
A closed system with no substances entering or leaving.
what are le Chatelier's principle
The position of equilibrium shifts to try to cancel out any changes you make
What can have an effect on the position of equilibrium?
Concentration in solutions
Temperature
Pressure in the gases
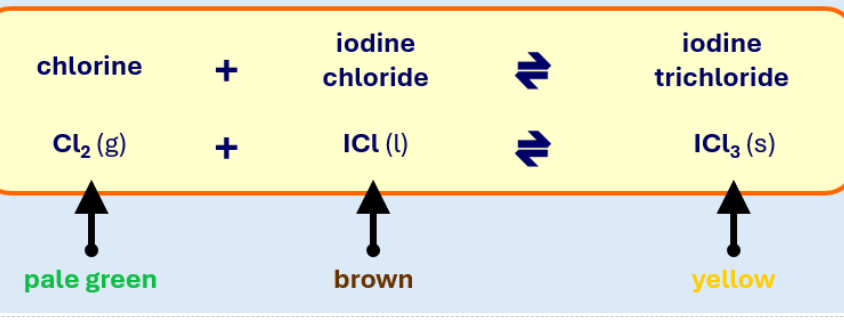
What effect will adding more Cl2 have on the colour of the mixture?
The green colour fades and the yellow increases
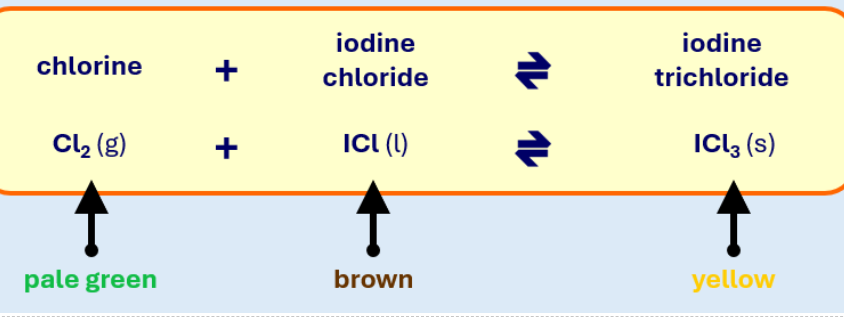
What effect will removing Cl2 have on the colour of the mixture and why?
the equilibrium shifts to the left producing more Cl2 and ICl so it will become more brown

What would you observe if you added water to this reaction?
Equilibrium shifts to the left
the colour will become less orange
What happens to the equilibrium position if temperature is increased?
It shifts in the endothermic direction to absorb the added heat.
Why does the system shift in the endothermic direction when temperature increases?
absorbs the heat added to reduce the added heat and oppose the change.
What happens to the equilibrium position if temperature is decreased?
It shifts in the exothermic direction to release heat.
Why does the system shift in the exothermic direction when temperature decreases?
To replace the lost heat and oppose the change.
What happens if pressure is increased in a gaseous equilibrium?
The system shifts to the side with fewer gas molecules.
Why does increasing pressure shift equilibrium to fewer gas molecules?
To reduce the pressure and oppose the change.
What happens if pressure is decreased in a gaseous equilibrium?
The system shifts to the side with more gas molecules.
Why does decreasing pressure shift equilibrium to more gas molecules?
To increase the pressure and oppose the change.
Does a change in pressure affect reactions where the number of gas molecules is the same on both sides?
No, there is no effect on the position of equilibrium.
What happens if the concentration of a reactant is increased?
The equilibrium shifts to the right to use up the added reactant.
What happens if the concentration of a product is increased?
The equilibrium shifts to the left to use up the added product.
What happens if the concentration of a reactant is decreased?
The equilibrium shifts to the left to replace the lost reactant.
What happens if the concentration of a product is decreased?
The equilibrium shifts to the right to replace the lost product.
What does it mean when equilibrium shifts to the right?
The reaction favors the forward direction and makes more products.
What does it mean when equilibrium shifts to the left?
The reaction favors the reverse direction and makes more reactants.
What effect do catalysts have on the position of equilibrium?
Catalysts do not affect the position of equilibrium.
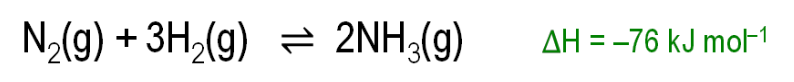
Why is a low temperature good for ammonia yield?
Because the forward reaction is exothermic, so low temperature favours ammonia formation.
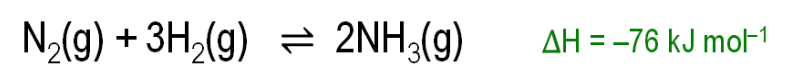
Why is a low temperature bad for industrial ammonia production?
Because it makes the reaction too slow, as particles have less energy and collide less often.