04 Metals and Ceramics 1
1/45
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
46 Terms
How is a crystalline material defined?
Material whose molecules are arranged in a highly ordered, periodic structure over large atomic distances
The arrangement within a period is called “unit cell“
All metals and ceramics form crystalline structures under normal solidification conditions
How is non-crystalline material defined?
(Also amorphous) material which lacks a systematic and regular arrangement of molecules
How is lattice defined?
3D array of points coinciding with atom positions (sphere centers)
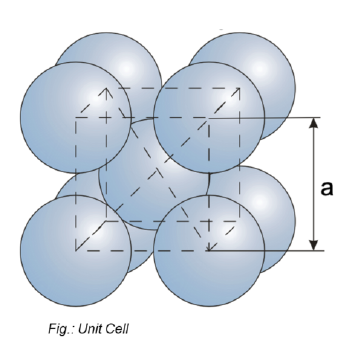
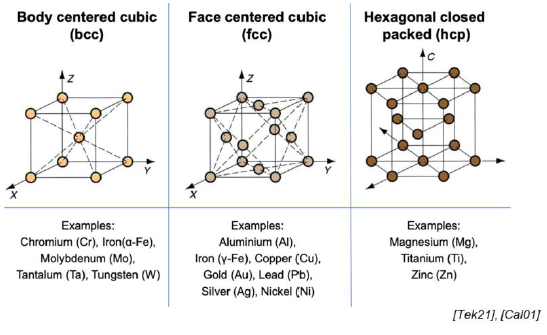
What are the common lattice structures? (3)
Body centered cube (BCC)
Face centered cube (FCC)
Hexagonal closed packed (HCP)
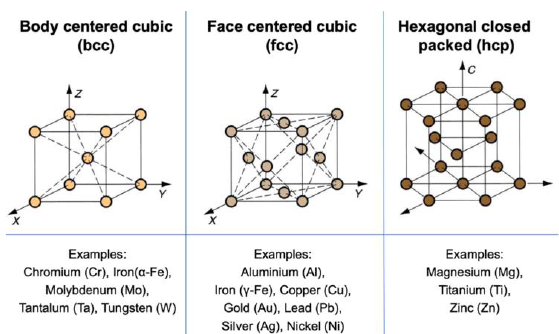
What does BCC mean? Give examples (Hint: apparently harder)
Body centered cube
Cr
Alpha-Fe
Mo
Ta
W
What does FCC mean? Give examples (Hint: apparently most ductile)
Face centered cube
Al
Gamma-Fe
Cu
Au
Pb
Ag
What does HCP mean? Give examples (Hint: apparently somewhere inbetween)
Mg
Ti
Zn
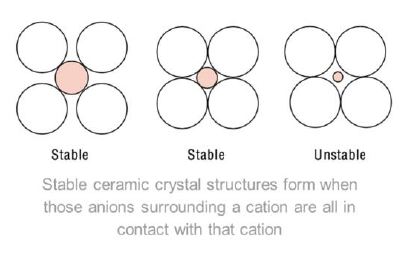
How are ceramics formed?
The whole crystall has to be electrically neutral
Out of electrically charged ions instead of atoms like for metals
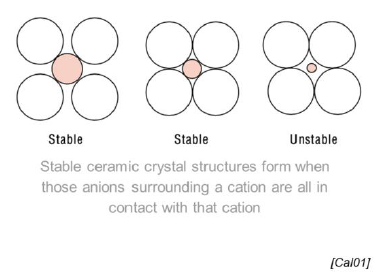
Radii of Cations / Radii of Anions < 1 (a cation surrounded by many anions)
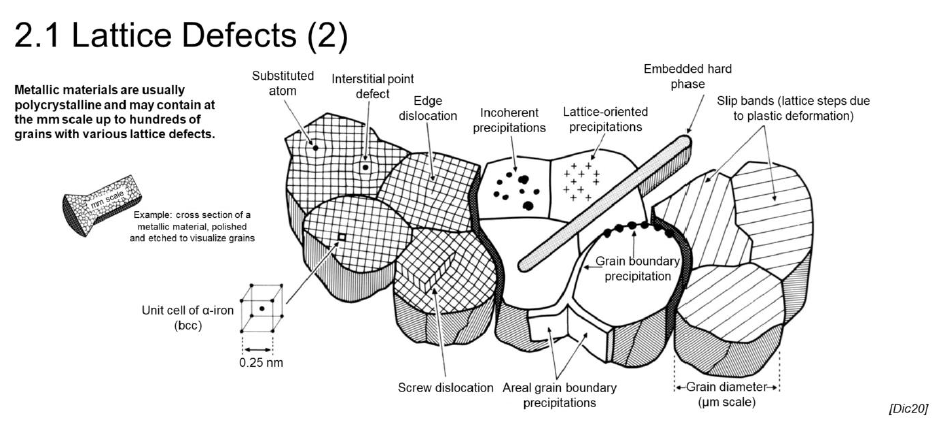
What are lattice defects?
Imperfections on form or composition within the lattice structure of a material
Can be you used to “tailor“ the material
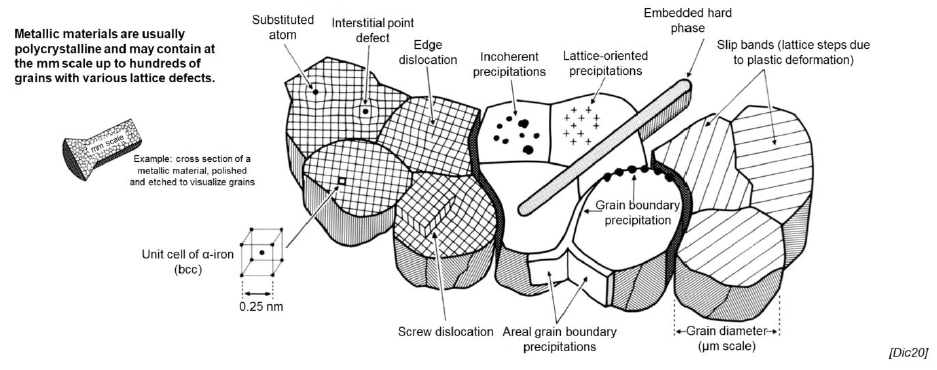
Which types of lattice defects are there? (4)
0-dimensional: point defects
1-dimensional: linear defects/dislocations
2-dimensional: interfacial defects, stacking faults, grain boundaries
3-dimensional: precipitations, impurities, voids
When do point defects occur? (4)
An atom form the crystal is crwoded into an interstitial site (self-interstitial atom (same species))
Foreign atoms crowded in lattice (substitutional atoms) or in interstices (interstitial atoms (interstitial atom))
Lattice sites no ocupied (vacancies)
Solid solution (larger amount of a second species dissolved in the lattice) (alloys)
HINT: extra atoms, atoms missing, foreign atoms
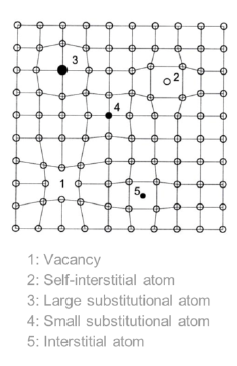
What kind of defects are alloys?
0-dimensional (point defects), namely solid solutions
Which types of 1-dimensional defects are there? (2)
Edge dislocation
Screw dislocation
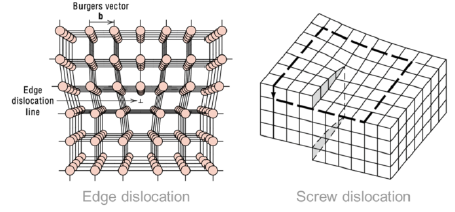
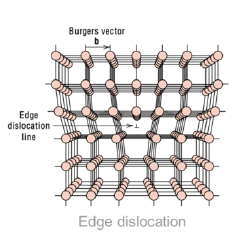
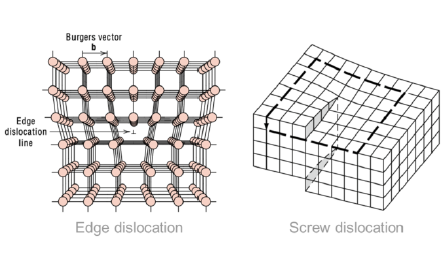
What are edge dislocations?
1-dimensional defect around which some atoms are misaligned
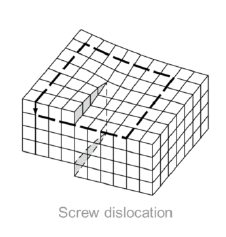
What are screw dislocations?
1-dimensional defect caused by a shear stress, deforming the lattice structure
How can linear defects be recognized?
They star and end at the surface of the crystal, or
form a loop within the crystal
Why can linear defects be useful?
Dislocations can be created with little enrgy inputs, giving metals their ductility
Dislocation interference strengthens the material (strain hardening)
How can interfacial defects be recognized?
Separate regions of the material with different crystal structures or crystallographic orientations
Include external surfaces, grain boundaries, stackin faults, phase boundaries
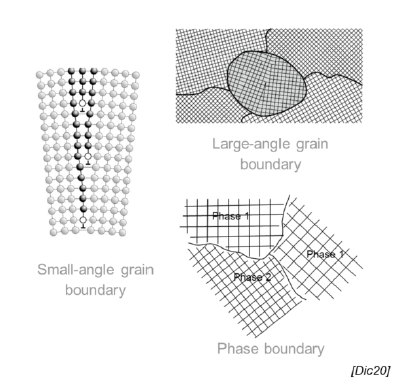
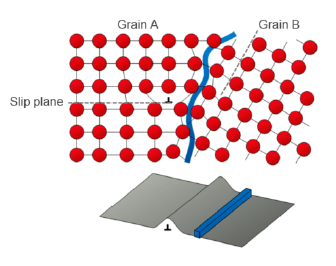
What is a grain boundary?
Meeting point of areas of material with different orientations of the lattice structure
How are interfacial defects formed? (2)
Different crystallization speed leads to different size and form of grains
Different grains touch eventually, stopping the crystallization
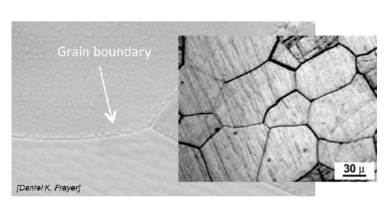
Why can grain boundaries be useful?
They block dislocation migration → better strength behavior
What are 3-dimensional defects?
Bulk defects/macroscopic defects like pores, cracks or inclusions
Normally introduced during processing and fabrication
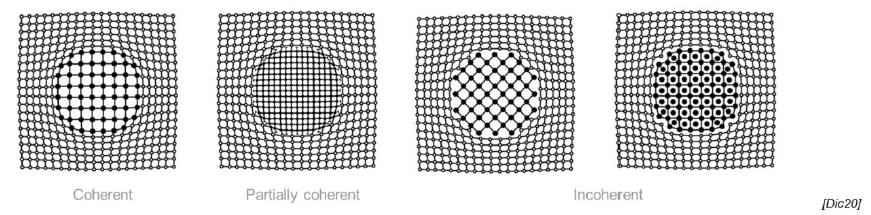
Which types of 3-dimensional defect are there? (3)
Coherent, partially coherent, incoherent
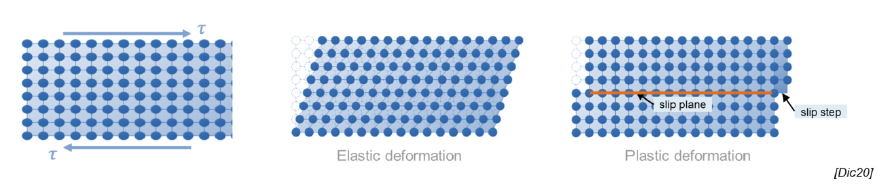
What is an elastic deformation?
Temporary deformation due to application of stress, after which the material returns to its original shape
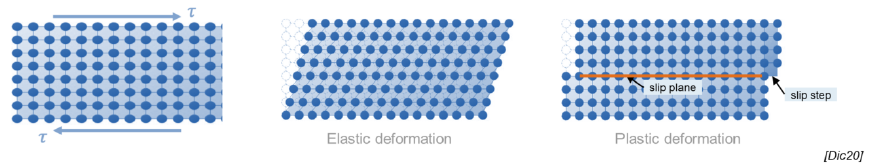
What is a plastic deformation?
Deformation in which rupture and reformation of interatomic bonds lead to permanent deformation
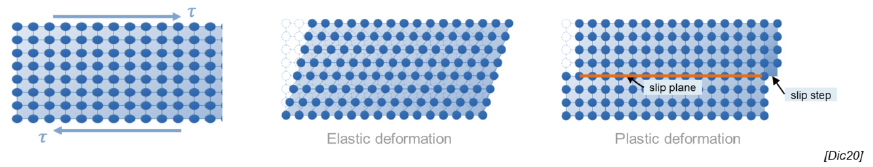
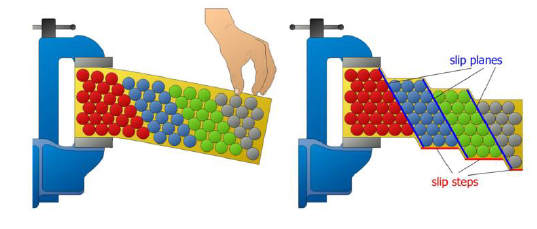
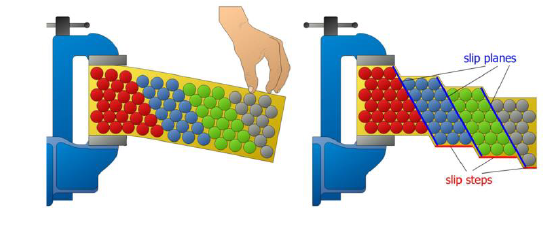
Def: slip, slip plane, slip steps
Process by which plastic deformation is produced by a dislocation motion
Slip plane: crystallographic plane traversed by the slip
Slip steps: result of slip on the slip steps
What is a slip system?
Combination of slip plane and slip direction
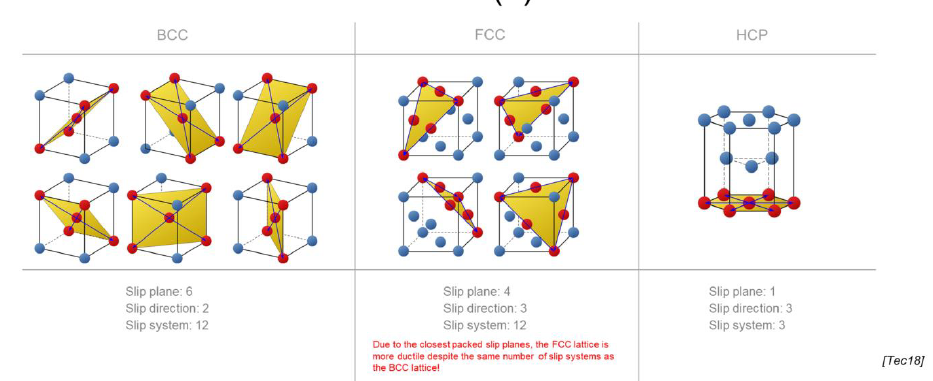
What is the relation between the number of slip systems and the ductility of a meterial?
Increased number of slip planes leads to a greater deformability
How are unit cells ordered from less ductile to more ductile?
HCP (Ti, Zn) < BCC (alpha-Iron) < FCC (Cu, Al, Au)
Why is FCC more ductile than BCC?
Despite having the same amount of slip systems, FCC slip systems are more densely packed than BCC
What happens during a dislocation? (2)
Regions in tensile, compressive and shear stress arise
The overall sum of dislocations still forms a perfect crystall
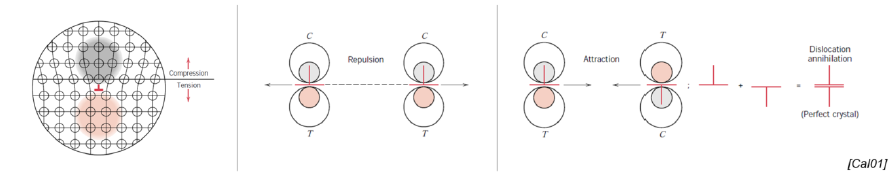
What happens during a tensile test wrt dislocation formation?
They are created in the elastic region, migrate in the Lüders region and pile up towards the ultimate strength (stagnation of the curve)
Dislocation interference hindered deformation until failure of the material
What strenghtening mechanisms are there? (3)
Solid-solution strengthening
Strengthening by grain size reduction
Strain hardening
What is Solid-solution strengthening?
A strengthening mechanism
Foreign atoms are introduced in the lattice, hindering dislocation migration
For both metal and cereamics
Basically, an alloy
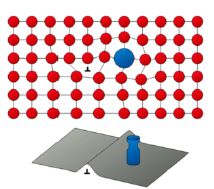
What is Strengthening by grain size reduction?
Large number of grains via size reduction
Grain boundaries hinder dislocation migration by
a) direction change of dislocation at grain boundary
b) discontinuity of slip planes
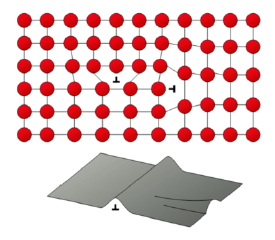
What is Strain Hardening?
Ductile materials become harder via plastic deformation
Already created deformations hinder new dislocation migration
Achieved through cold working
SQ: What is the difference in molecular structure between crystalline and non-crystalline materials?
Crystalline materials: periodically ordered and repetitive over large atomic distances
Non-crystalline materials: lack systematic and regular arrangement of molecules
SQ: Draw unit cells for FCC, BCC, and HCP crystal structures.
FCC: cube, atom in each corner, atom in the center of each face
BCC: cube, atom in each corner, atom in the very center of the cube
HCP: hexagonal prism, atom in each corner, atom in the center of each hexagonal face, atom traingle in the middle of the prism
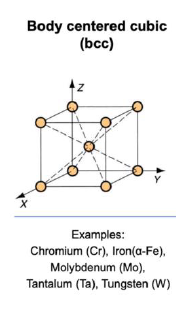
SQ: What is the relationship between the unit cell edge and the atomic radius of a BCC structure?
a = 4R/√3
SQ: What is the difference between metallic lattices and ceramic lattices?
Metal lattice composed of atoms, ceramic lattice composed of cations and anions (rC / rA < 1
Ceramic lattice more complex
Ceramic crystall must be electrically neutral
SQ: What are the lattice defects? Why are they important?
Various imperfections present in all solids, classified into 0, 1, 2, 3 dimensional defects
They enable material tailoring by changing material properties such as conductivity, ductility, hardness, etc.
SQ: Describe edge and screw dislocation motion from an atomic perspective
Edge dislocation: slip along slip plane, leading to misalignment of atoms
Screw dislocation: deformation caused by a shear stress, expressed in terms of the Burgers Vector
SQ: How can materials be plastically deformed?
Applied stress leads to rupture and reformation of atomic bonds, so that material gets permanently deformed
SQ: What are slip planes and slip systems?
Slip planes: crystallographic plane traversed by a dislocation. Plane in which atoms displace in a plastic deformation
Slips system: combination of a slip plane and a slip direction
SQ: How many slip planes do the different lattices have?
FCC: 12
BCC: 12
HCP: 3
SQ: What are the different types of strengthening mechanisms and how do they work?
Solid-Solution Strengthening: inclusion of foreign atomic species in the material to hinder dislocation propagations
Strengthening by reduction of grain size: reduction of grain size leads increased amount of grains, which hinder migration of dislocations since grain boundaries induce a change of direction in propagation of the dislocation and discontinuity of the slip planes
Strain hardening: already existing dislocations hinder migration of new ones. Usually achieved through cold working