Enthalpy of Dissolution & Hydration & Size Mismatches
1/7
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
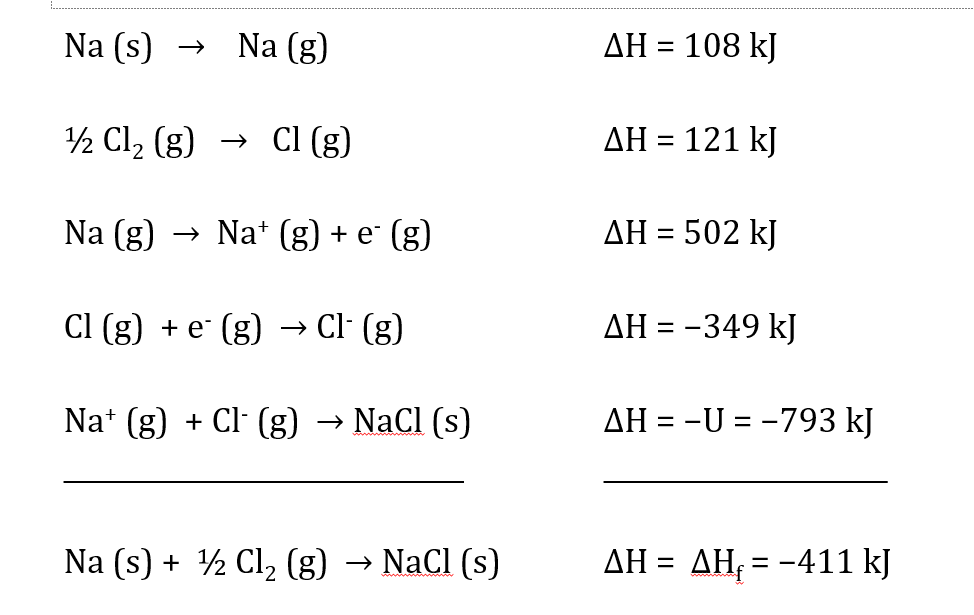
Calculate the enthalpy for formation of NaCl(s) if it takes 108 kJ to make a mole of sodium into gas, 242 kJ to split a mole of diatomic chlorine gas into chlorine gas, 502 kJ to remove an electron from Na(g), -349 kJ to ionize a mole of chlorine gas, and the lattice energy U of NaCl is -793 kJ.
Use enthalpy of formation of NaCl2(s) to explain why NaCl2(s) doesn’t really exist.
Very positive value = very endothermic = very unfavorable reaction
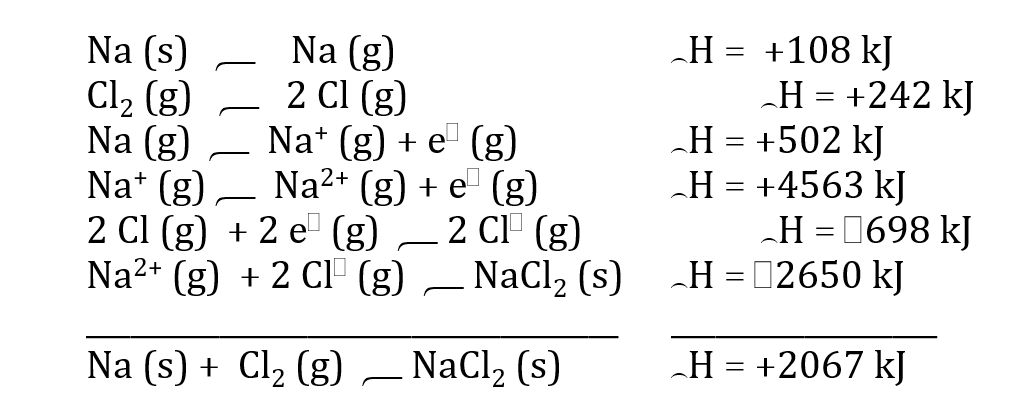
What two values do you have to add to get an ion’s hydration number?
primary hydration sphere and secondary hydration sphere
What is the primary number that influences the hydration of an ion?
charge density
As size mismatch increases, solubility of ionic compounds tend to ___ (increase/decrease).
increase
Alkaline earth carbonates tend to be ___ (more/less) stable as the size mismatch between the cation and the anion increases.
less
Ions with larger charge densities also tend to have more ___ (positive/negative) hydration enthalpies.
negative
Describe the difference that a catalyst makes in terms of the energy vs. reaction pathway graph.
A catalyst makes the activation energy smaller, making the hump shorter.