8-Substitution and Elimination Reactions
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
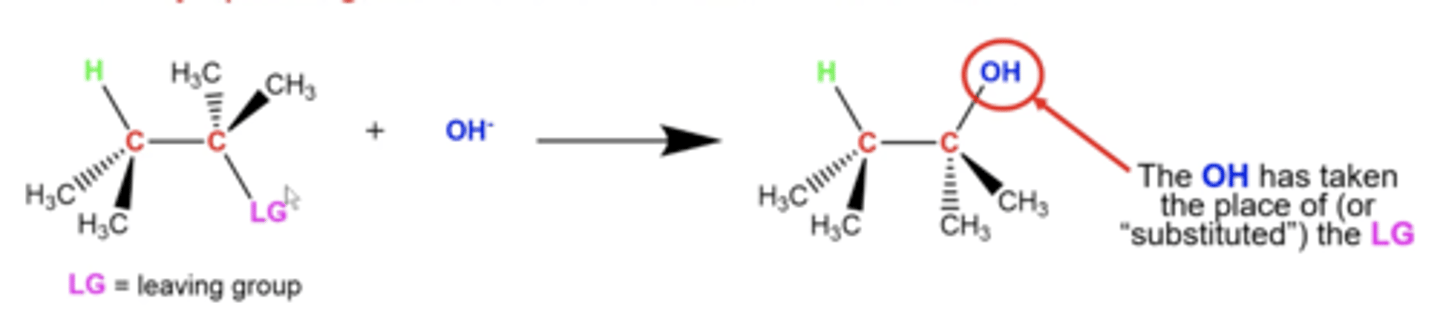
in general, what is the premise of substitution reactions?
Some nucleophile (the thing that does the attacking with its e-) will replace a leaving group in a molecule
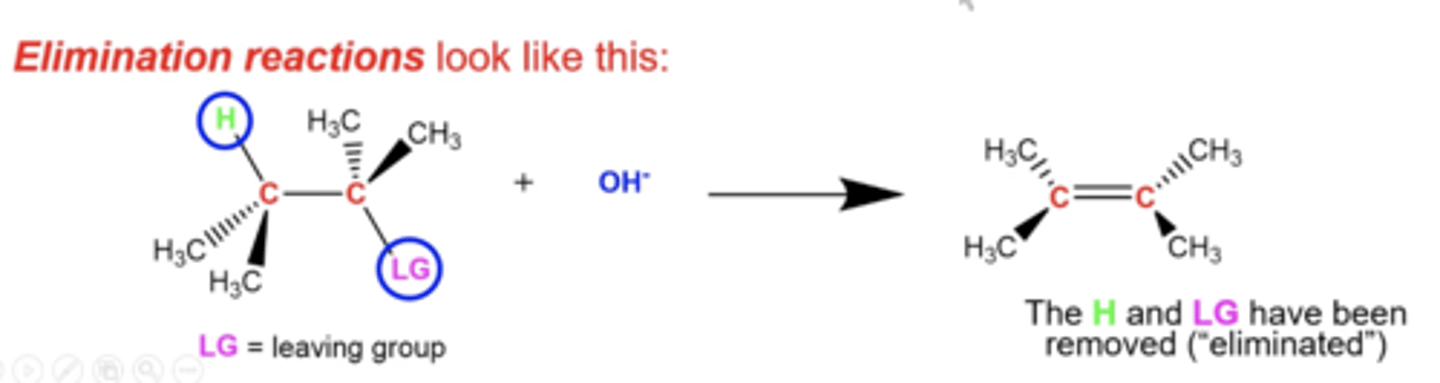
in general, what is the premise of elimination reactions?
the leaving group and some other group like H may be eliminated, sometimes to perform an oxidation reaction
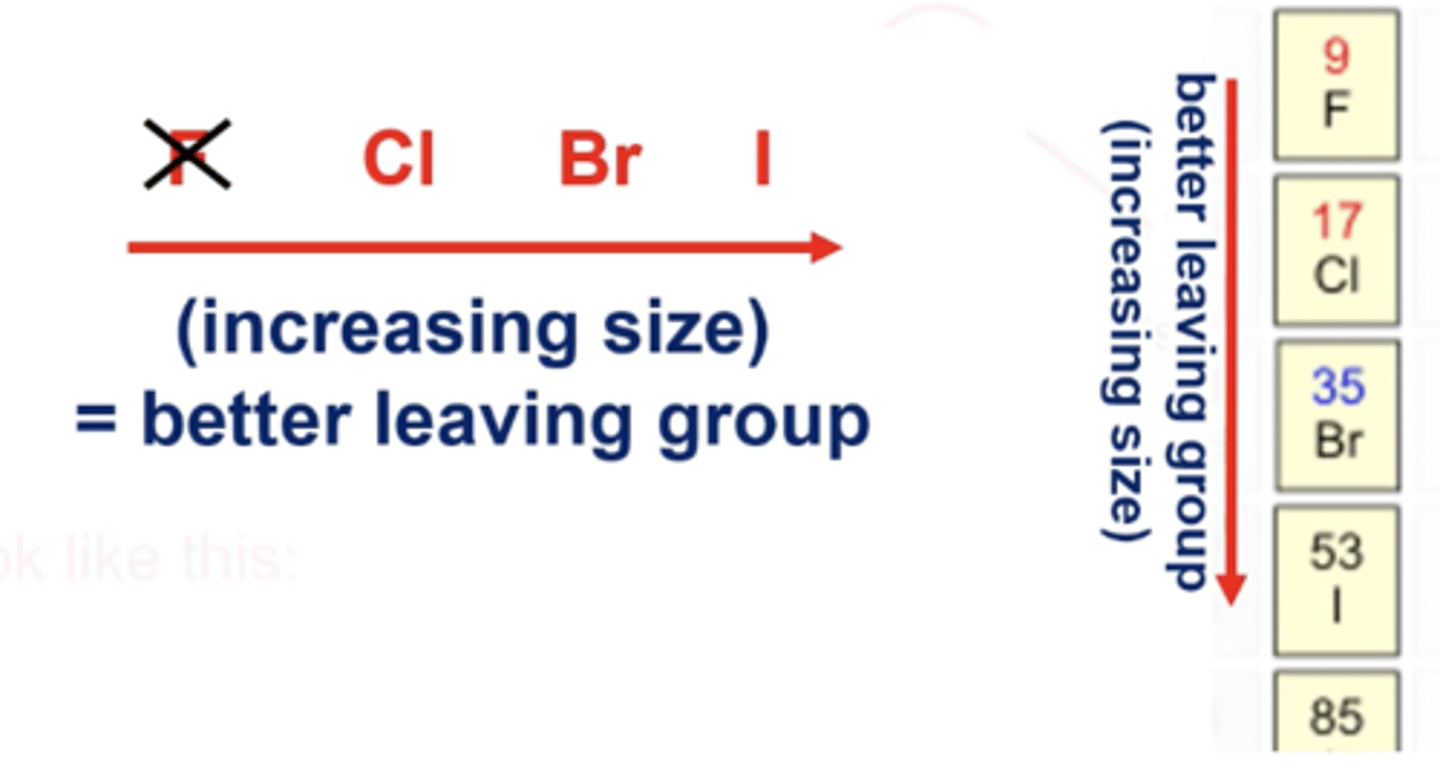
how does size correlate to how good a leaving group is?
halogens are common leaving groups
-the larger the leaving group, the better. This is because of a longer C-halogen bond (we don't even really consider F a leaving group because it's so small)
what are some other leaving groups you may encounter?
TsO, TfO, MsO
aka tosylates, triflates, and mesylates
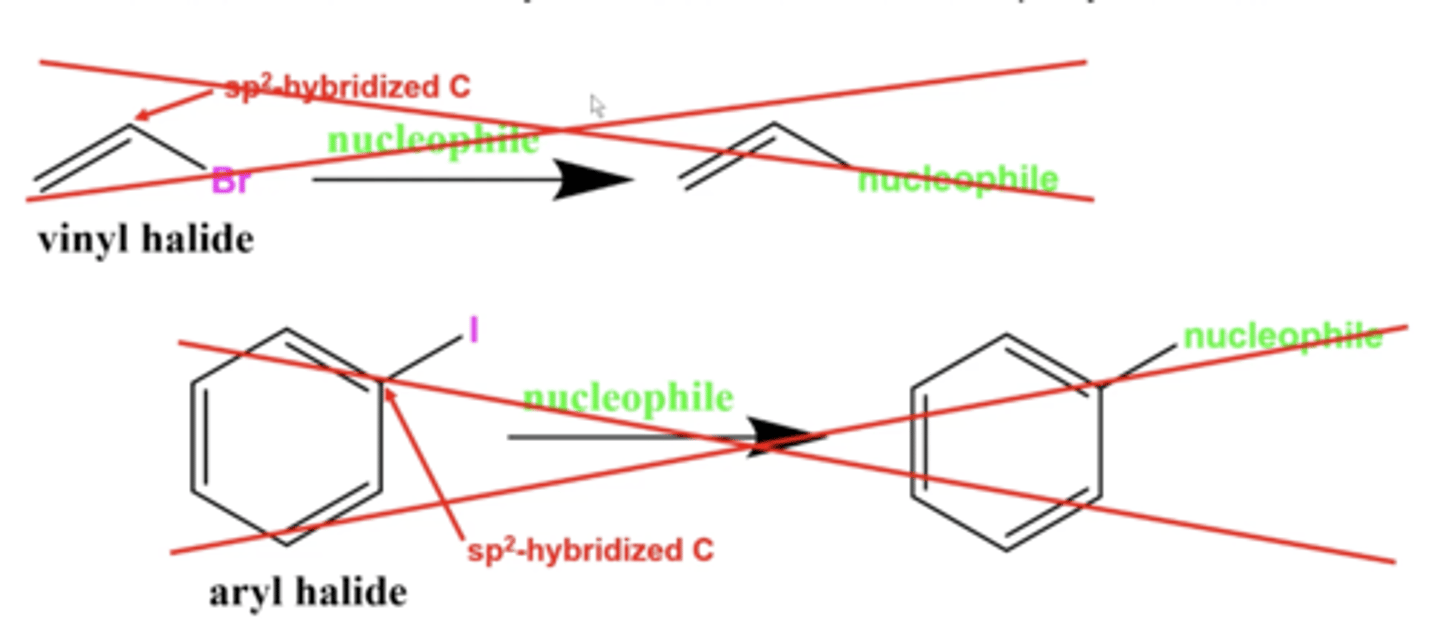
what kind of carbon will you never have a leaving group/substitution reaction on?
carbons with leaving groups on sp2 hybridized carbons
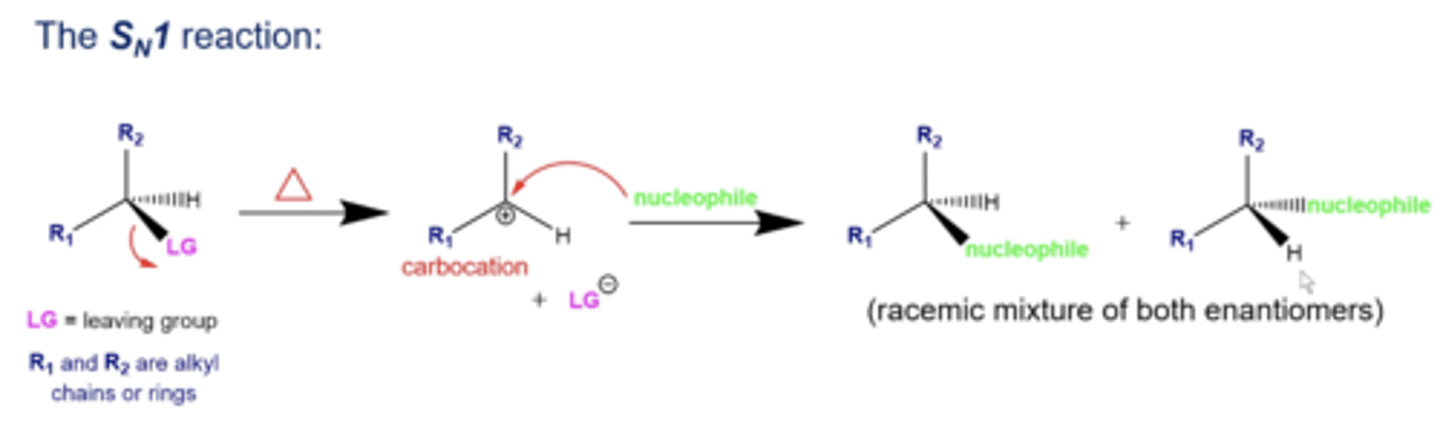
what does Sn1 stand for?
"Substitution nucleophilic unimolecular"
-this means there's only one reactant involved in the rate determining step (formation of a carbocation)
in general, what happens in Sn1 reactions?
1. a reactant with a leaving group encounters heat
2. upon adding heat, the carbon-L.G. bond breaks, with the L.G. taking the e- it was sharing with the C, forming a carbocation
3. a nucleophile will now attack the carbocation to form a racemic mixture of two enantiomer products
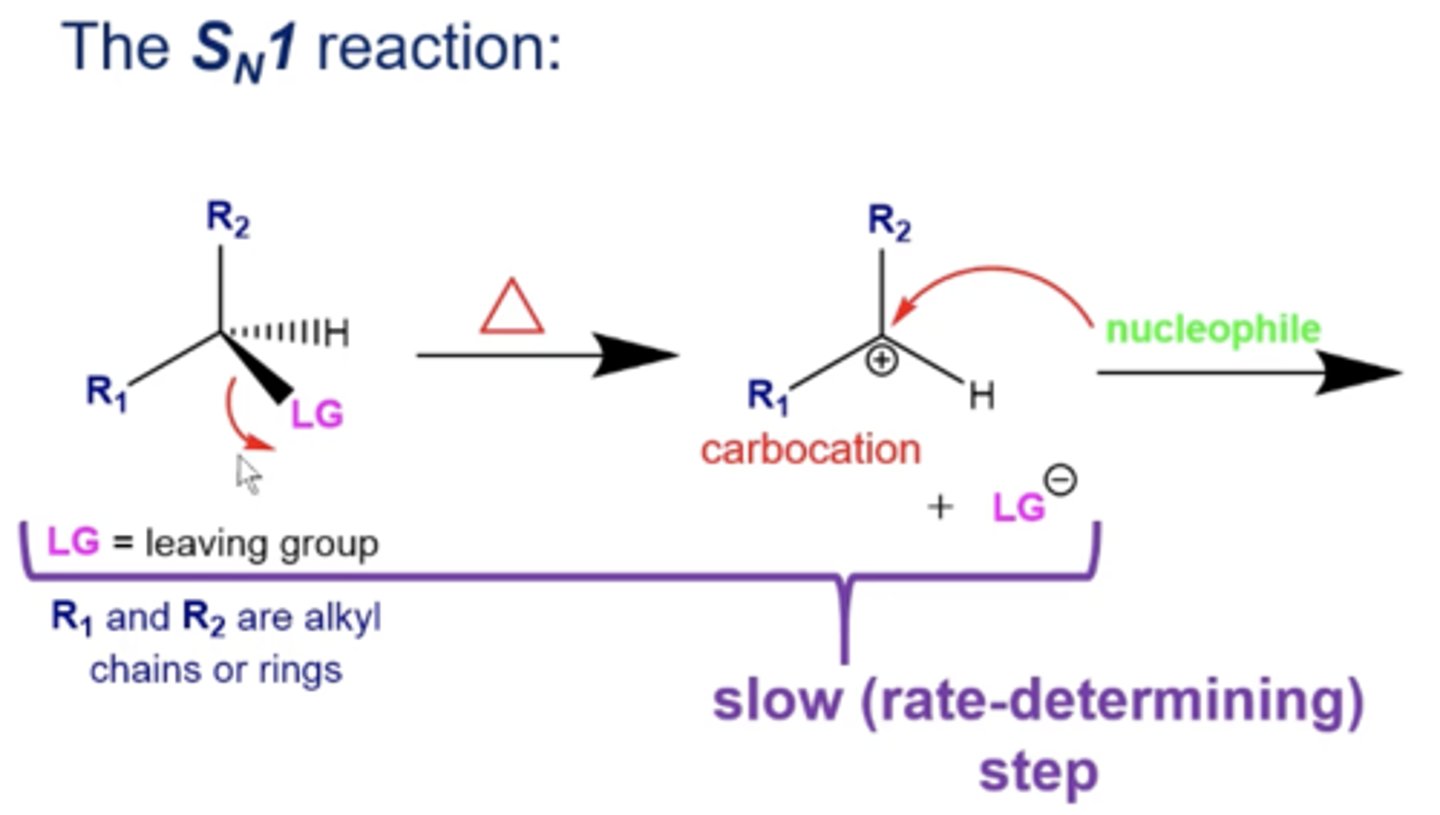
in Sn1 reactions, what is the speed of the formation of a carbocation?
the formation of the carbocation is SLOW and it's the RATE DETERMINING STEP of an Sn1 reaction
what is the rate law for an Sn1 reaction?
Rate = k[electrophile]
-this is because only the electrophile is involved in the rate-determining step of forming a carbocation
how does the stability of a carbocation affect how fast an Sn1 reaction proceeds?
the more stable a carbocation (electrophile), the faster the reaction will proceed
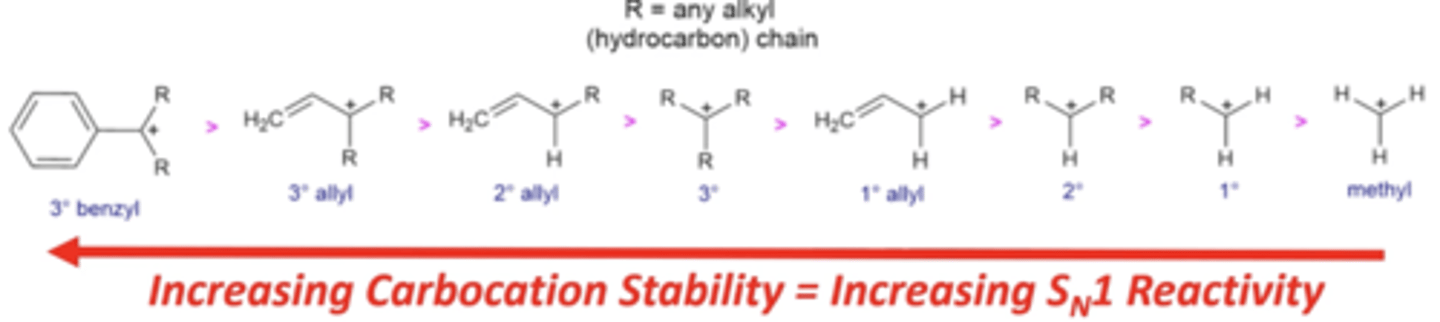
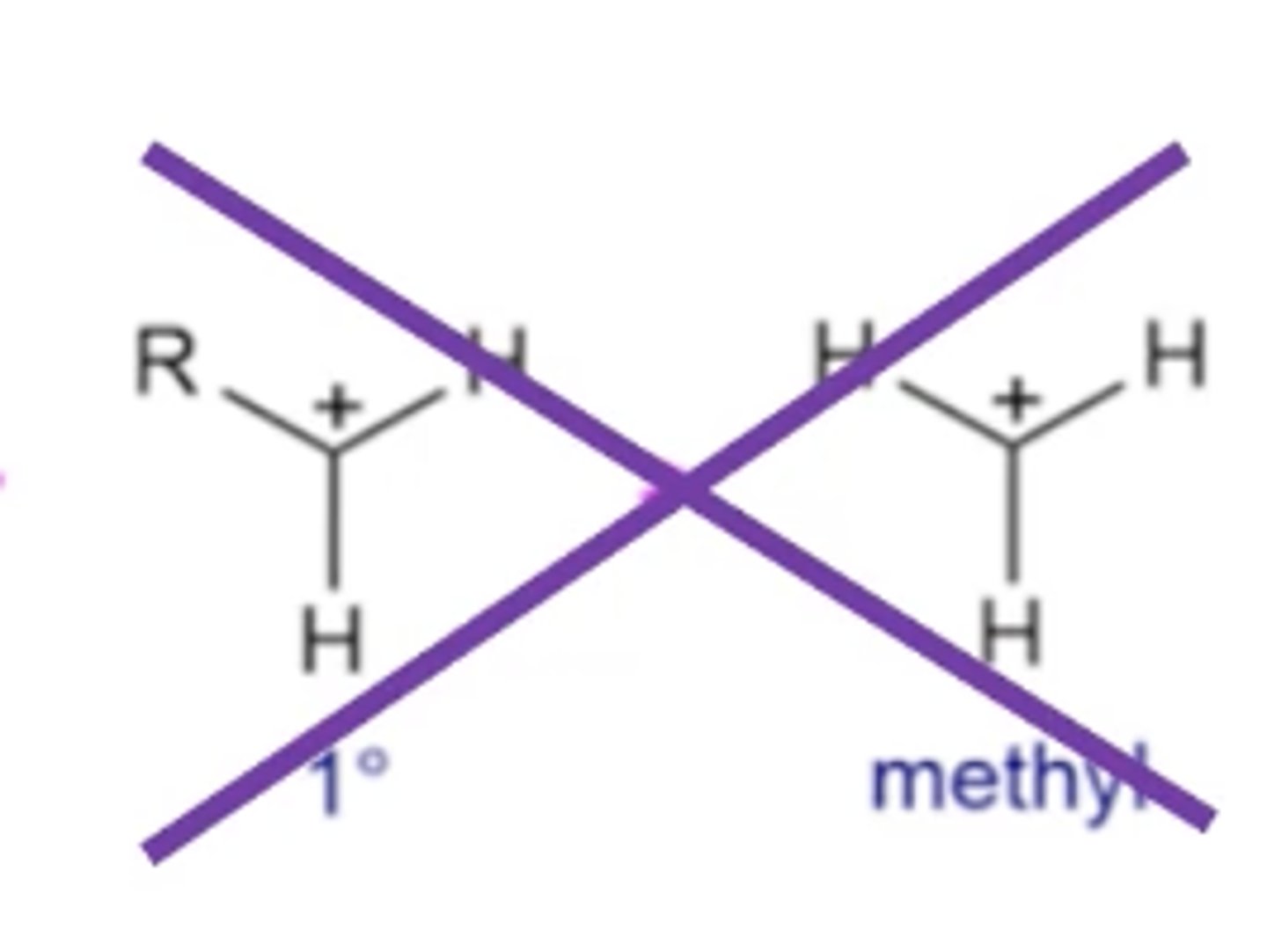
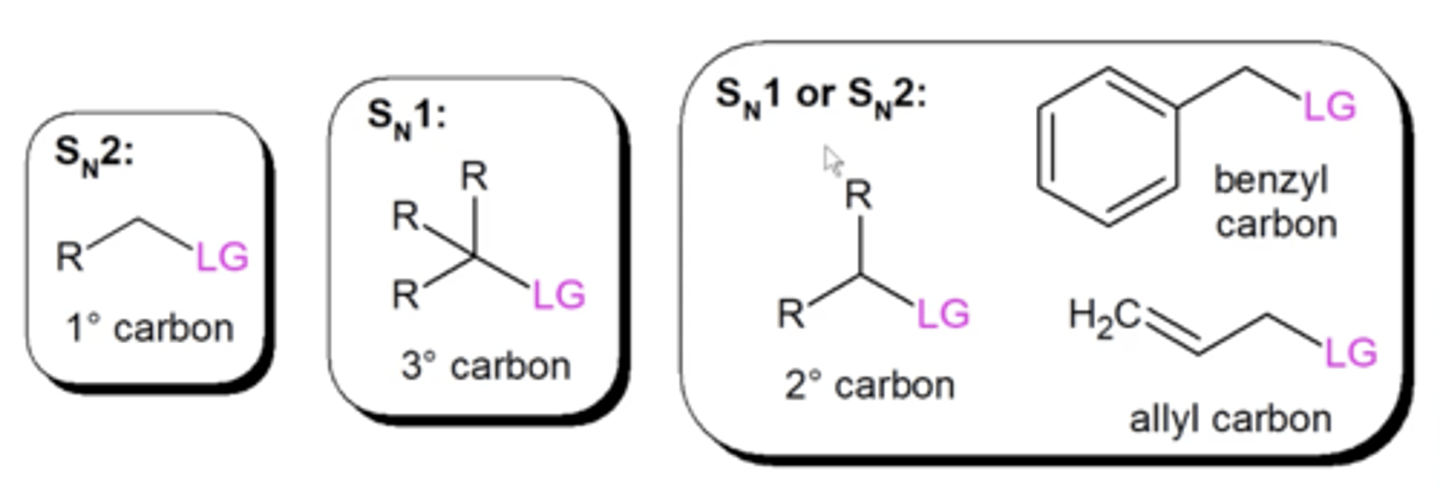
which molecules/electrophiles will never undergo Sn1 reactions?
primary carbocations and methyl carbocations
why do Sn1 reactions only involve weak nucleophiles?
strong nucleophiles are too reactive to allow a carbocation to form
why do Sn1 reactions produce racemic enantiomer mixtures?
because the nucleophile can attack the carbocation from either side (front or back)
how do rearrangements occur in Sn1 reactions vs. Sn2 reactions?
because carbocations form in Sn1 reactions, they can undergo rearrangement (hydride shifts, methyl shifts, rearrangements)
-because Sn2 reactions don't form carbocations, they can't undergo rearrangement
in general, what happens in Sn2 reactions?
1. a reactant with a leaving group encounters a very strong nucleophile
2. the nucleophile will attack the carbon bonded to the L.G. and kick the L.G. off in the same step
there's no formation of a carbocation!
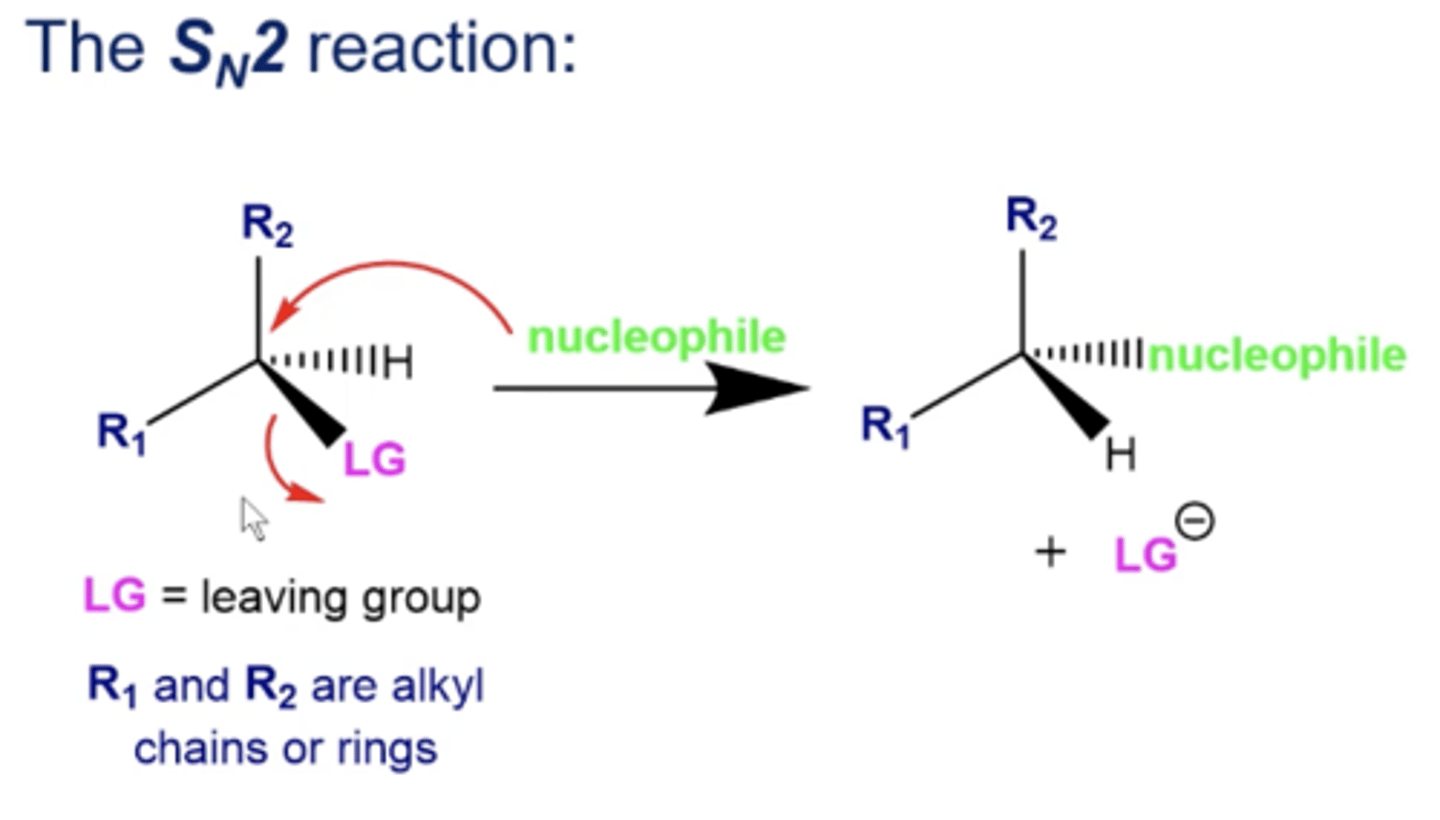
what does Sn2 stand for?
"substitution nucleophilic bimolecular*
-the electrophile and nucleophile are both involved in the rate-determining step (the L.G. being replaced by the nucleophile)
what is the rate law for Sn2 reactions?
rate = k[Nucleophile][Electrophile]
how does the substitution of the substrate electrophile affect the reactivity of Sn2 reactions?
single methyl groups and primary carbon groups are the most reactive substrates
-tertiary carbons don't even undergo Sn2 reactions
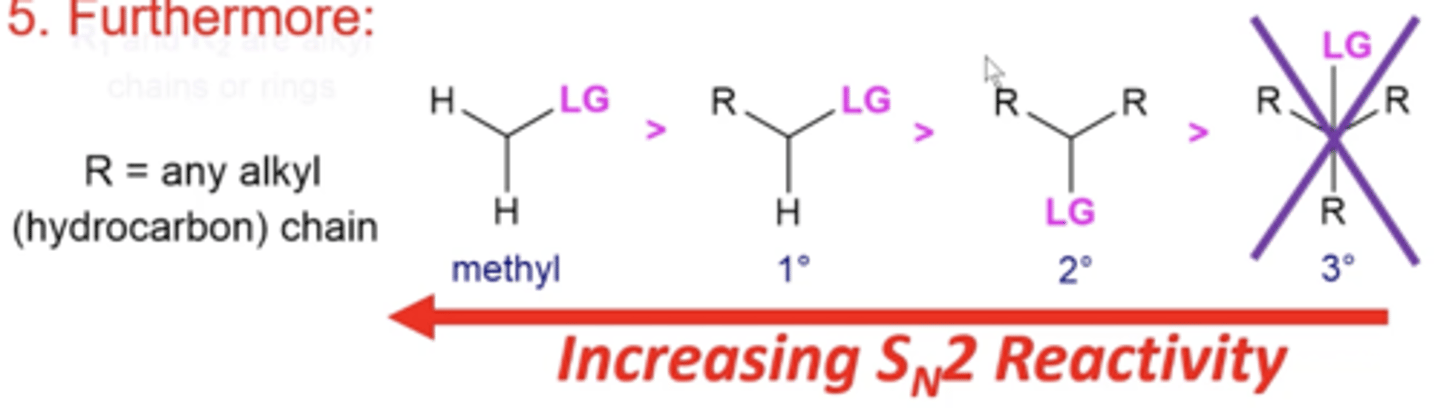
why are less substituted carbons more reactive in Sn2 reactions?
they're less sterically hindered and easier to be attacked by a nucleophile
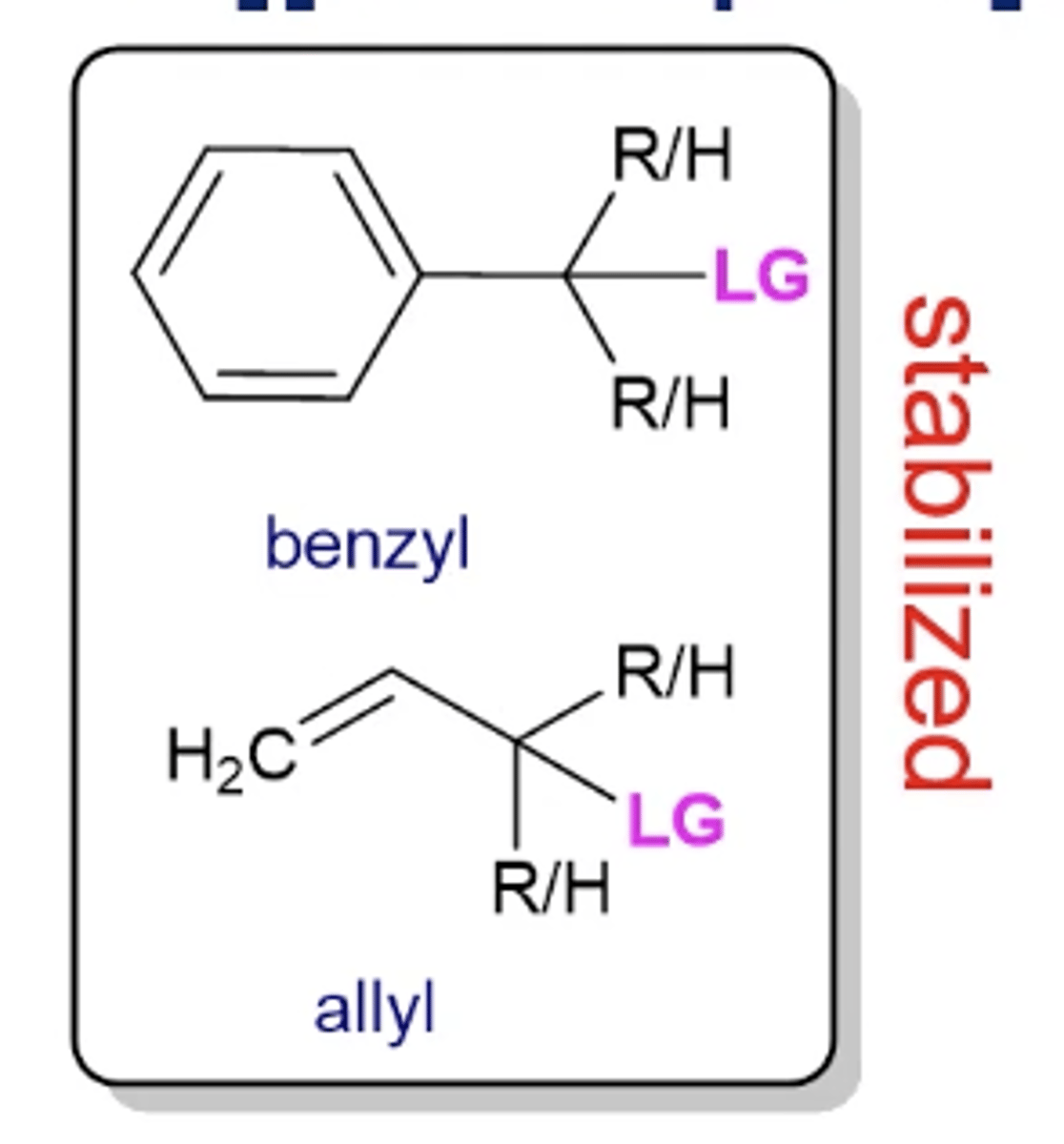
will stabilized carbocations like benzyl and allyls undergo Sn1 or Sn2 reactions?
even though these look like they're tertiary carbons, due to resonance, they can perform Sn2 reactions
it depends on the strength of the nucleophile
how does the nucleophile attack in Sn2 reactions?
the nucleophile does a backside attack, forming a transition state that is a pentacoordinate, with a central carbon being approximately sp2-hybridized
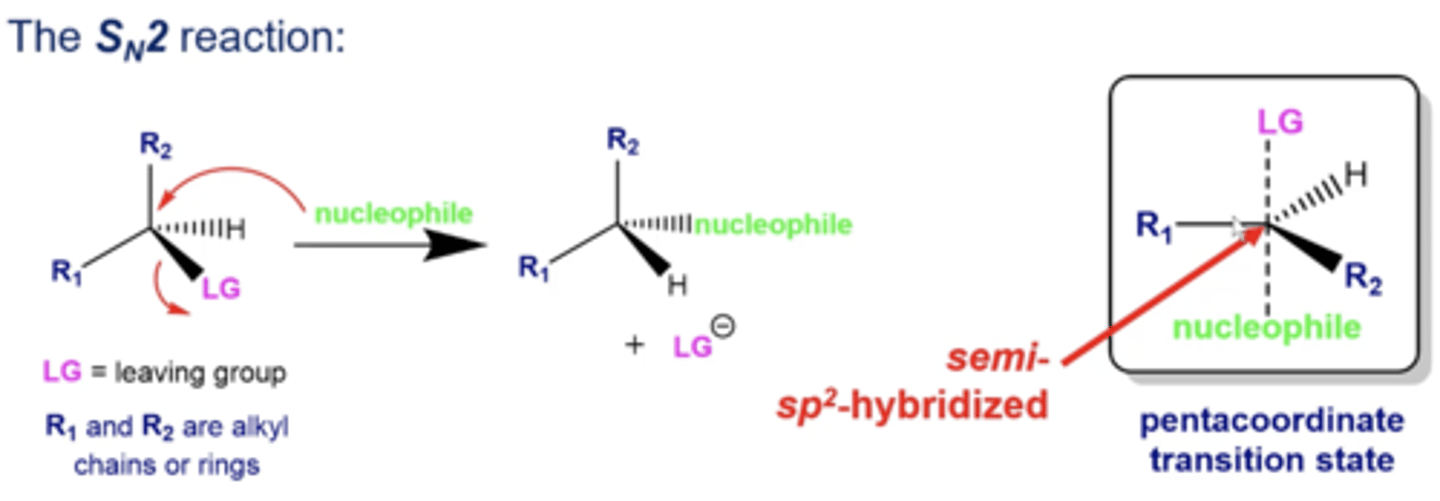
what type of nucleophile do you need in an Sn2 reaction?
strong nucleophiles!
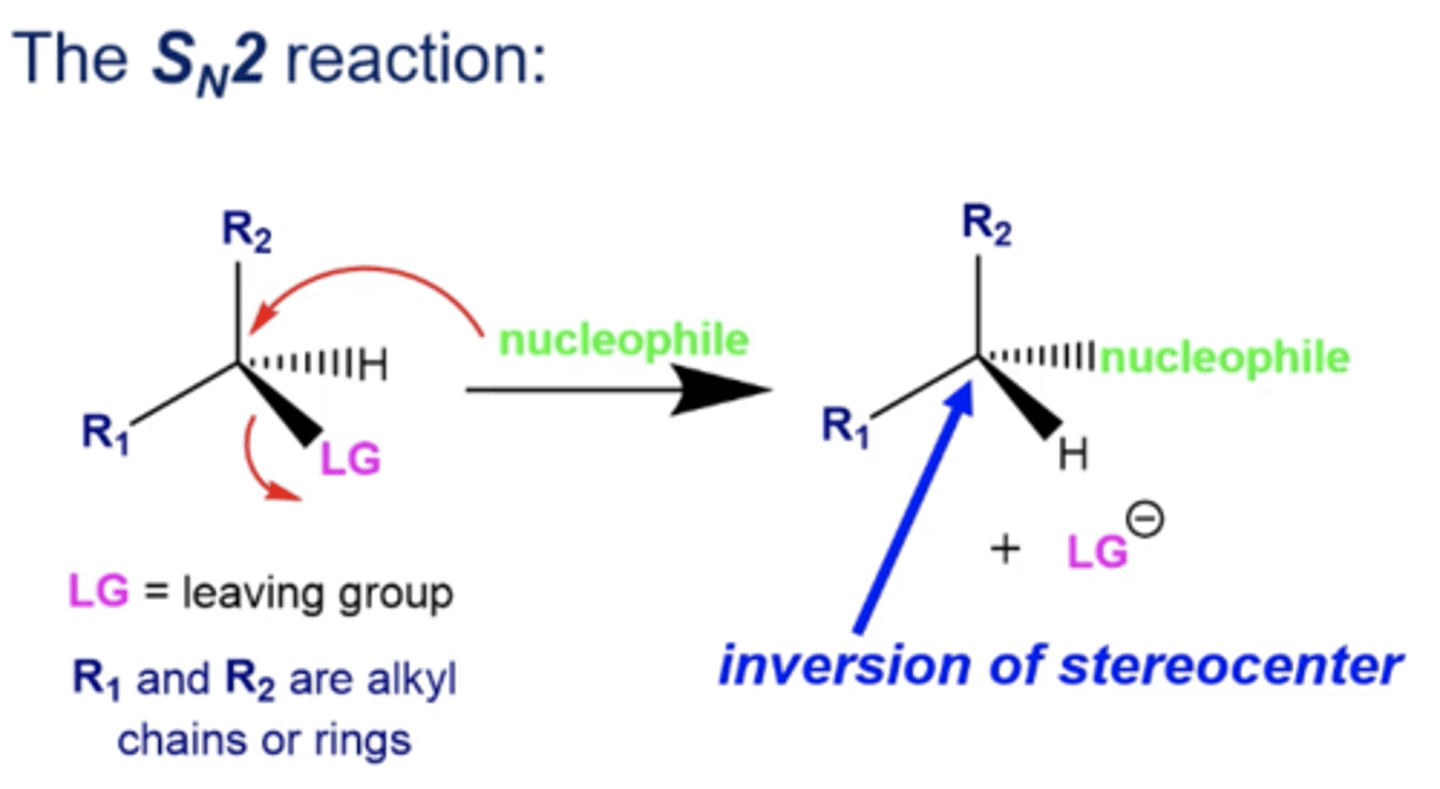
how does stereochemistry change during Sn2 reactions if your electrophile has a stereocenter?
Sn2 reactions will invert that stereocenter's stereochemistry
how do you choose between Sn1 and Sn2 reactions?
1. is the carbon that's bonded to the L.G. primary, secondary, or tertiary?
2. is the nucleophile strong or weak?
how can you determine if a nucleophile is strong or weak?
strong nucleophiles have localized negative charges (no resonance)
-resonance-stabilized negative charges are weak nucleophiles
what are some strong nucleophiles (for Sn2 reactions)?
Metal-CN, Metal-OR, Metal-OH, Metal-SR, Metal-NR2, Metal-R
Metal: Li, Na, K, Mg
R: alkyl or H

what are some weak nucleophiles (for Sn1 reactions)?
RCO2-, HOR, H2O, HSR, HNR2
-these O, S, or N only have lone pairs, but are not as strong as negative charges
R: alkyl or H
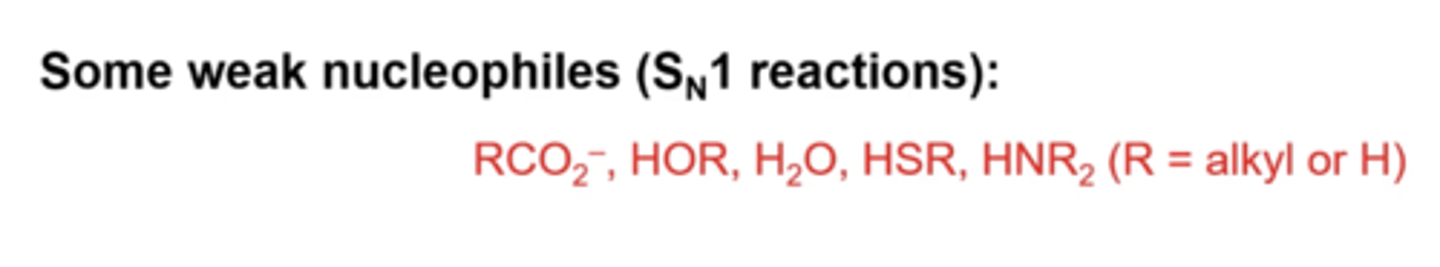
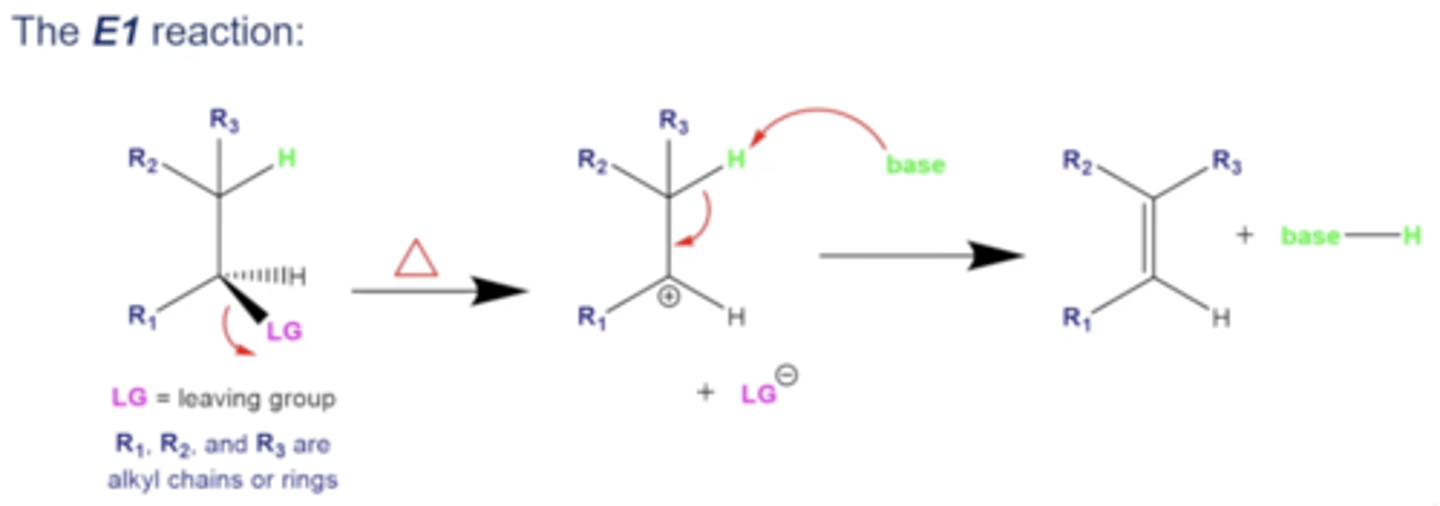
in general, what happens in E1 reactions?
1. a molecule with a leaving group is treated with heat
2. the leaving group leaves, taking its e- and leaving a carbocation in its place
3. a base will rip a hydrogen off of the neighboring carbon, forming a C-C double bond with the carbocation, ultimately forming an alkene
-the base is now protonated in solution
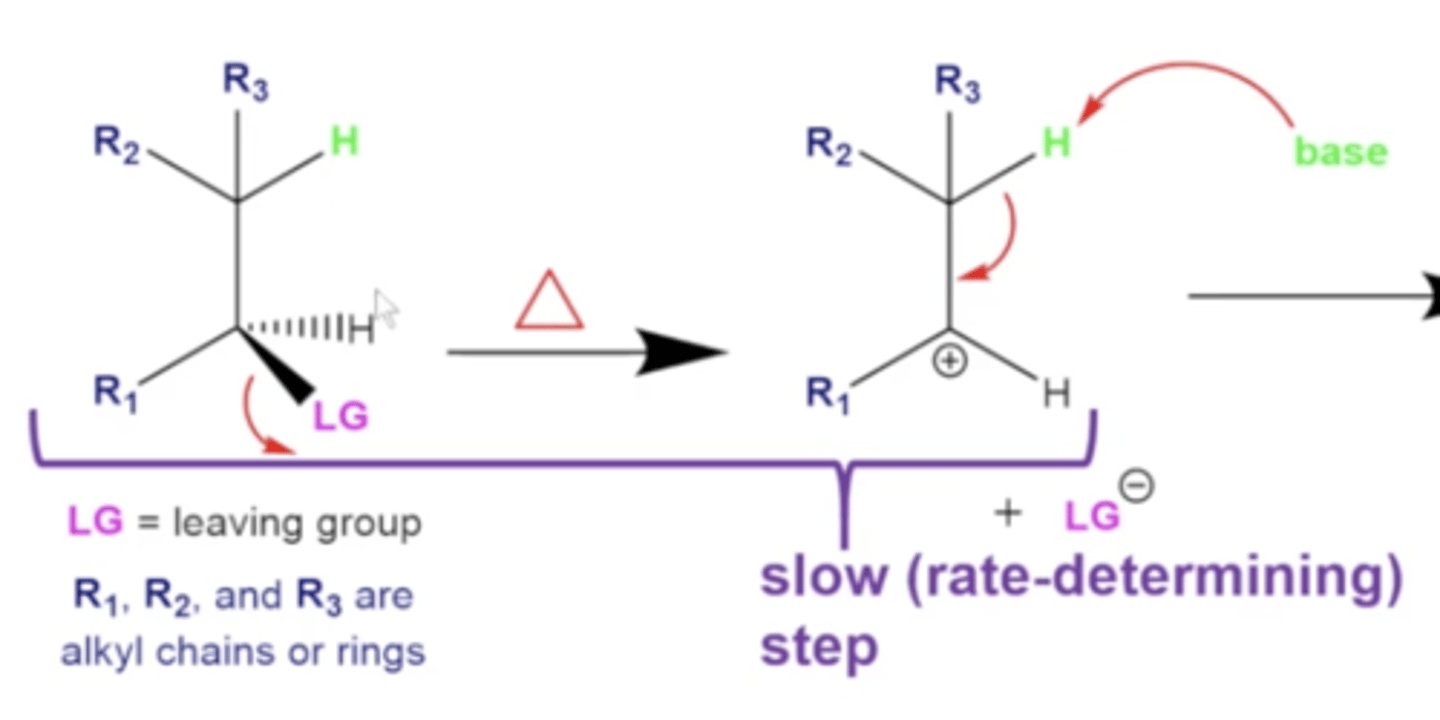
what is the rate-determining step in an E1 reaction?
the formation of the carbocation once the leaving group leaves
what does E1 stand for?
"elimination unimolecular"
-only the electrophile is involved in the rate-limiting step
what is the rate law for E1 reactions?
same as Sn1:
rate = k[electrophile]
how does the stability of a carbocation affect how fast an E1 reaction proceeds?
the more stable a carbocation (electrophile), the faster the reaction will proceed
-tertiary benzyls and allyls will be the fastest
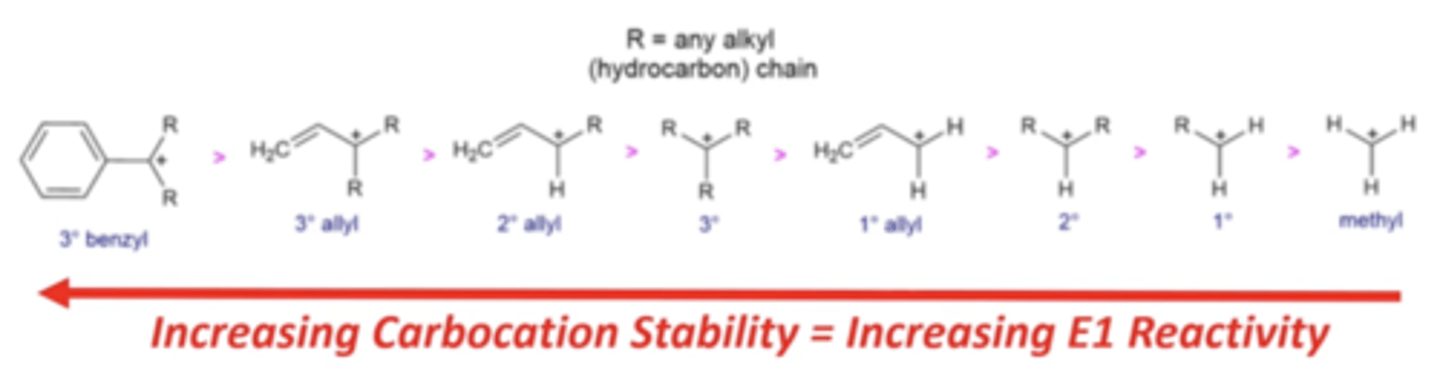
which electrophiles do not undergo E1 reactions?
primary and methyl electrophiles forming carbocations
which bases do E1 reactions involve?
WEAK bases, because strong bases are too reactive to allow a carbocation to form
which alkenes will E1 and E2 form? (E or Z?)
unless you use a sterically bulky tert-butoxy base, they will form the more substituted C=C bond (aka E-alkene)
-this is called Zaitsev's rule
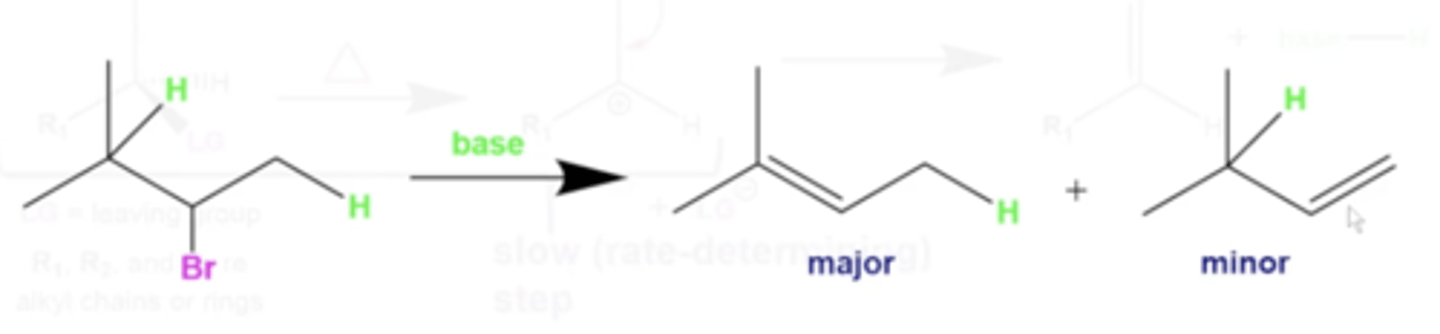
in general, what happens during an E2 reaction?
1. a molecule containing a leaving group reacts with a base, causing the base to simultaneously strip a hydrogen from a neighboring carbon and push the L.G. off
-this forms an alkene product with a hydrogenated base and negatively charged L.G. in solution
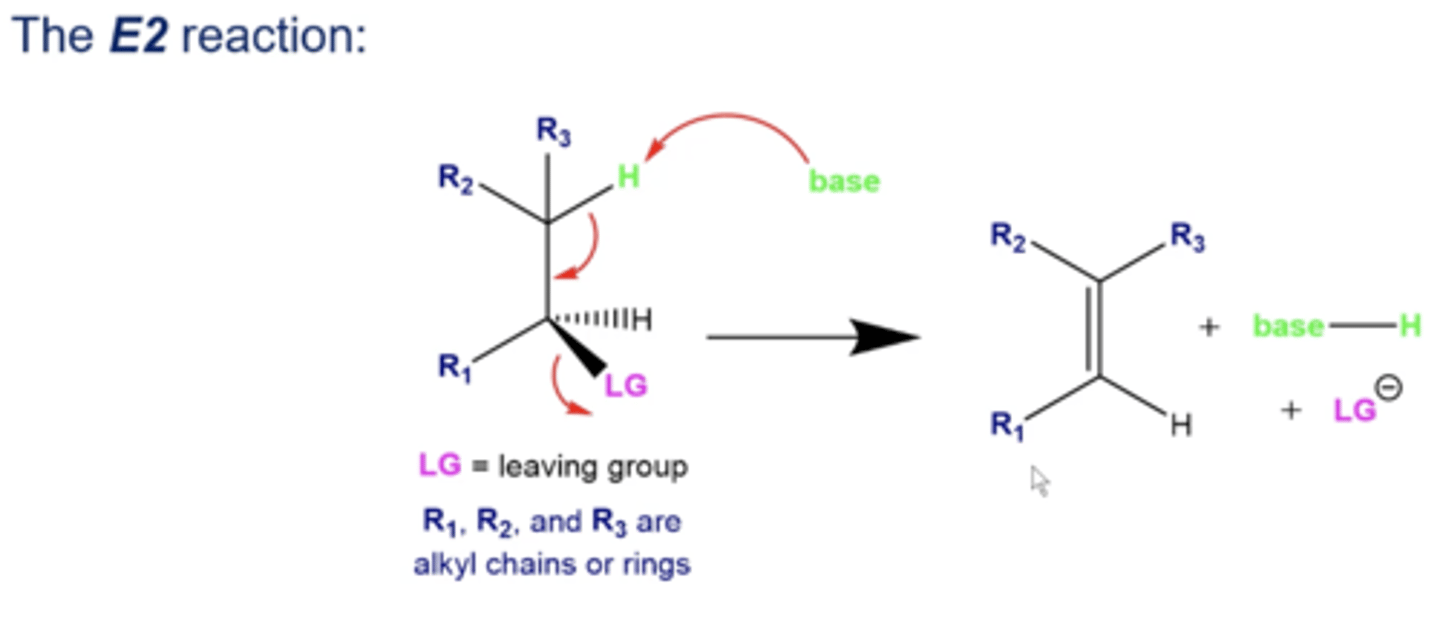
what does E2 stand for?
"elimination bimolecular"
-both the electrophile and the nucleophile (base) is involved in the rate-determining step (deprotonating and kicking L.G. off)
what is the rate law for E2 reactions?
same as Sn2:
rate = k[electrophile][base/nucleophile]
how does the substitution of an electrophile affect the reactivity of an E2 reaction?
(same as E1 rxns)
the more substituted the the carbon with the L.G., the more reactive the electrophile due to Zaitsev's rule to produce E alkenes
-methyls cannot undergo E2 reactions because they can't form C=C
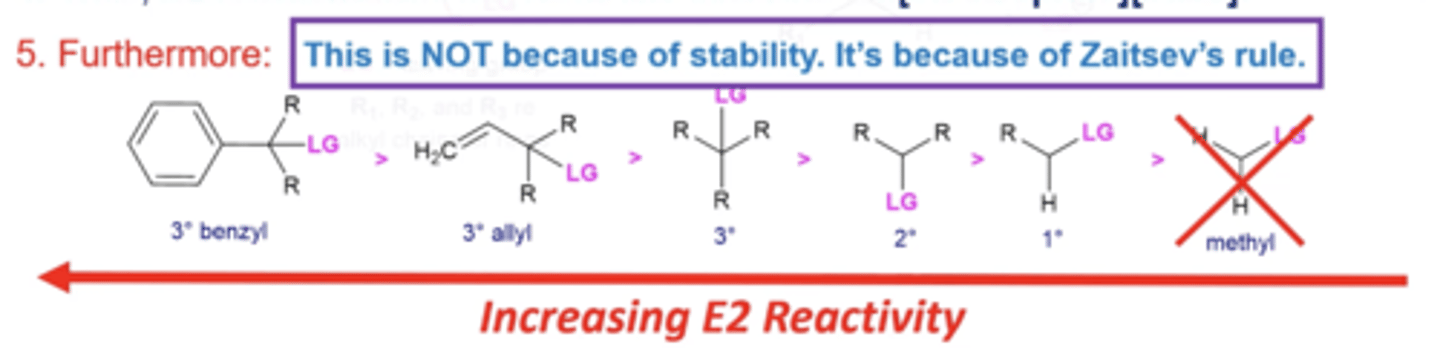
what about Zaitsev's rule makes more substituted electrophiles so favorable for E2 reactions?
the more C's coming off of a C=C, the more stable the alkene, thus the more carbons around the L.G. carbon, the more substituted alkene
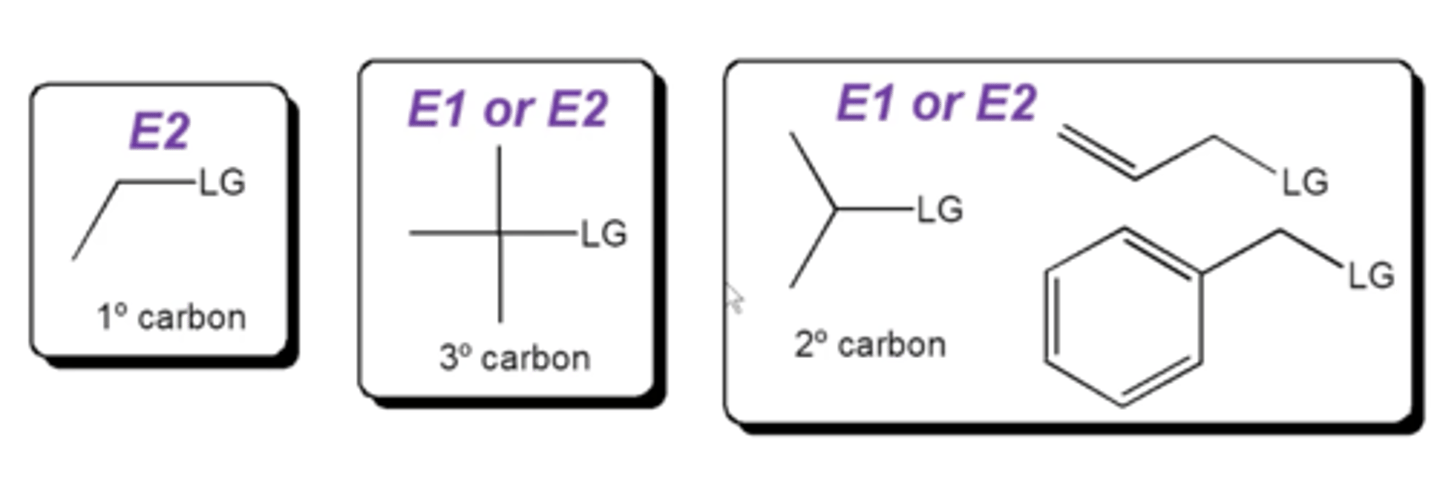
how do you choose between E1 and E2 reactions?
1. is the carbon that's bonded to the L.G. primary, secondary, or tertiary?
2. is the base strong or weak?
what are some strong bases (for E2 reactions)?
bases that have negative charges:
Metal-OR, Metal-OH, Metal-NR2
metal: Li, Na, K, Mg
R: alkyl or H

what are some weak bases (for E1 reactions)?
bases that have negative charges that are resonance-stabilized:
RCO2-, HOR, H2O, HNR2
R: alkyl or H

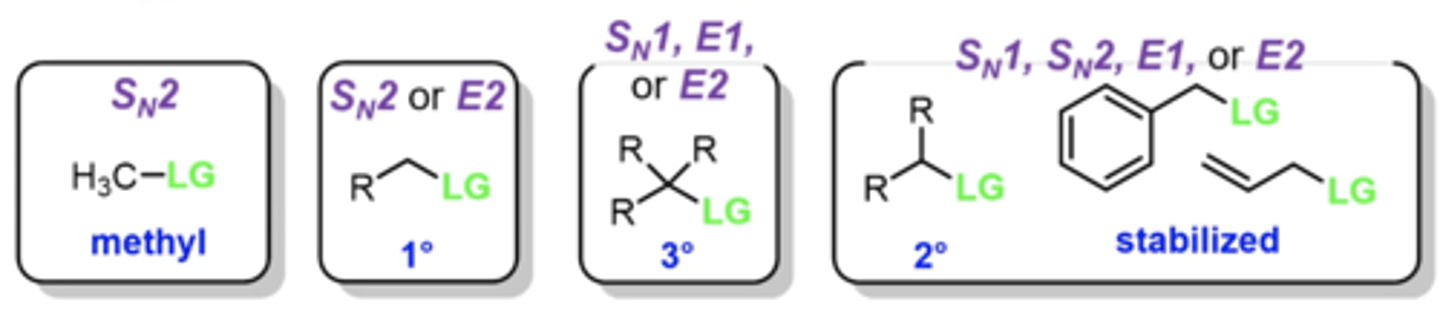
what is the list of questions to ask yourself to differentiate between Sn1, Sn2, E1, or E2?
1. does the question specifically say "substitution" or "elimination", or specify a specific pathway?
2. is the carbon bonded to the L.G. a methyl, primary, secondary, or tertiary carbon? (if methyl, it must be Sn2. if primary, it must be Sn2 or E2. If tertiary, it can be Sn1, E1, or E2. if secondary, it can be any)
3. if you nucleophile/base strong or weak?
4. is your nucleophile/base a nucleophile or base?
how can you differentiate between nucleophiles and bases?
larger nucleophiles/bases tend to behave more as bases (E1/2) because they CAN'T fit as easily into the carbon bonded to the L.G. to do a substitution. They therefore prefer to deprotonate somewhere else
smaller nucleophiles/bases tend to behave more as nucleophiles because they CAN fit more easily into the carbon bonded to the L.G. to do a substitution
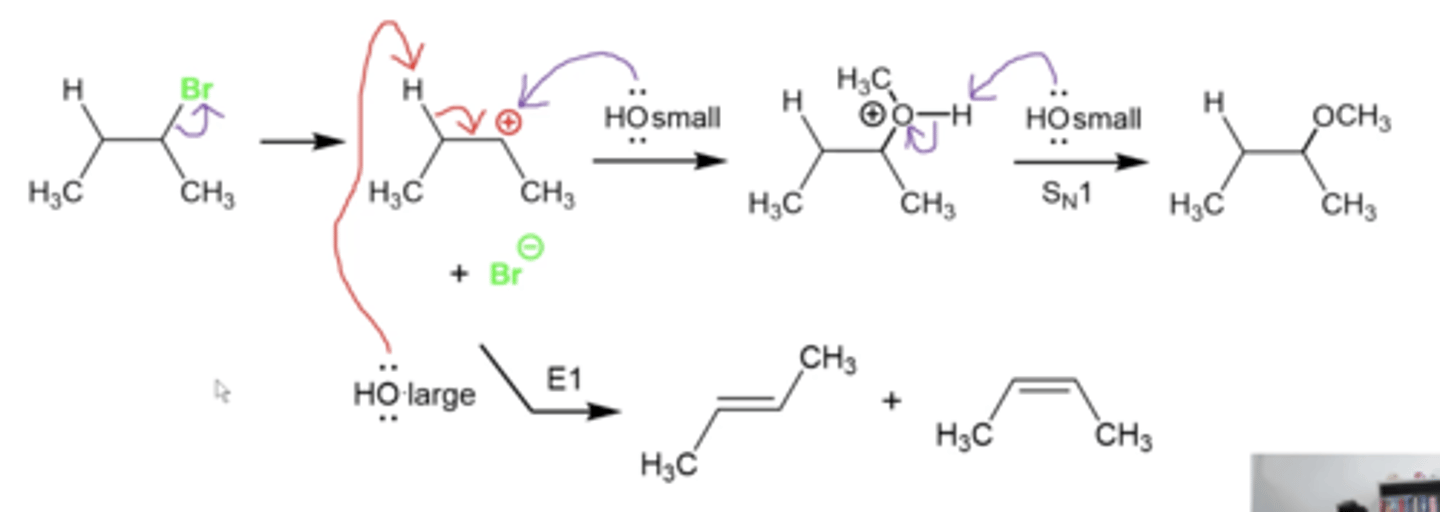
which bases are considered large or small?
any nucleophile/base that looks as big as ethanol or ethoxide (CH3CH2O-) or larger when drawn on paper, is a large BASE that will do an E reaction. Anything smaller will be a nucleophile and do an S reaction.

is acetate (CH3CO2-) a nucleophile or base?
although acetate (CH3CO2-) looks larger than ethanol, it will be a nucleophile to do S reactions
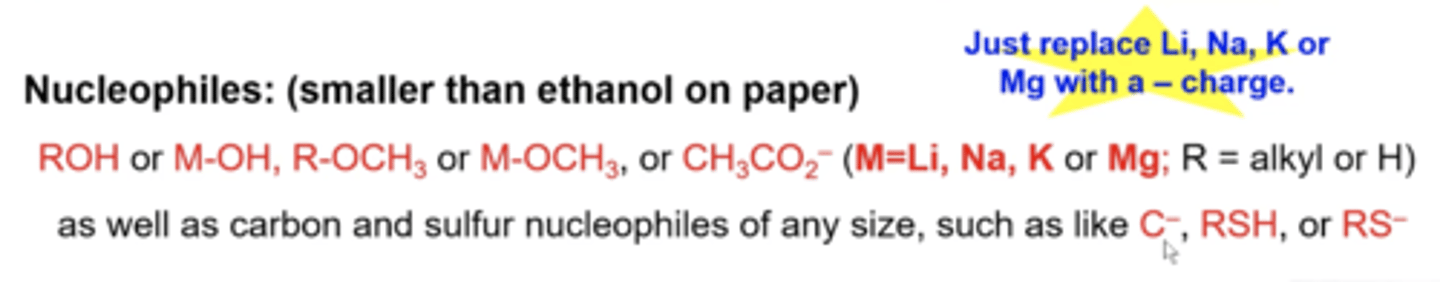
are negatively charge carbon and sulfur atoms nucleophiles or bases
they are usually nucleophiles, regardless of their actual size, and do S reactions
solvents that have H atoms bonded to an oxygen, nitrogen, or sulfur
ex. CH3OH, CH3CH2OH, acetic acid (CH3COOH), or H2O
protic solvent
solvents that do not have H atoms bonded to oxygen, nitrogen, or sulfur
ex.
DMSO
acetone
DMF
aprotic solvent
what reaction mechanism do protic solvents lean towards?
SN1, E1, and E2 reactions
HOWEVER, don't fixate as much on the solvent as the four questions detailed above!
what reaction mechanism do aprotic solvents lean towards?
Sn2 and E2 reactions
HOWEVER, don't fixate as much on the solvent as the four questions detailed above!
when a strong Sn2 nucleophile like CH3O- is paired with its conjugate acid (CH3OH) as solvent (aka a protic solvent), which mechanism will this reaction do?
Sn2
when will weak nucleophilic halides (Cl-, Br-, and I-) do Sn1 reactions instead of Sn2 reactions?
if you have the rare scenario where a halide is a nucleophile, which mechanism it does depends on the solvent:
-protic solvents do Sn1
-aprotic solvents do Sn2
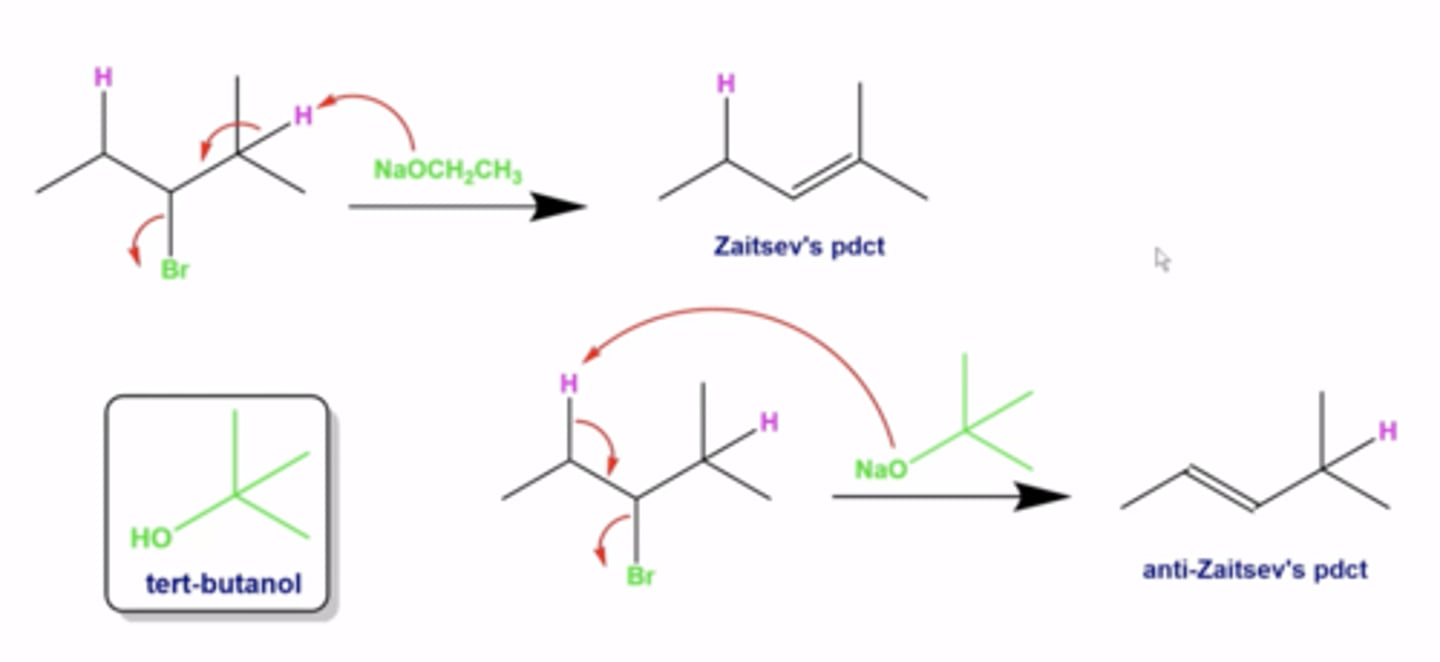
when will the Zaitsev product (the most substituted alkene) not be favored in elimination reactions?
-if you produce a conjugated diene, that is the favored pathway regardless of zaitsev's rule
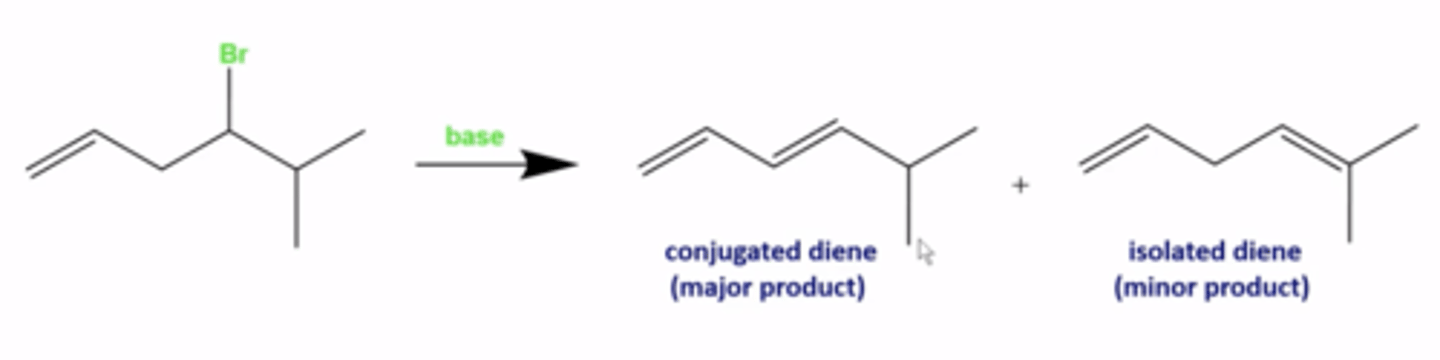
when will the Zaitsev product (the most substituted alkene) not be favored in elimination reactions (another exception)?
-if you use a bulky tert-butoxy base, the less substituted C=C bond product is favored (anti-Zaitsev product)
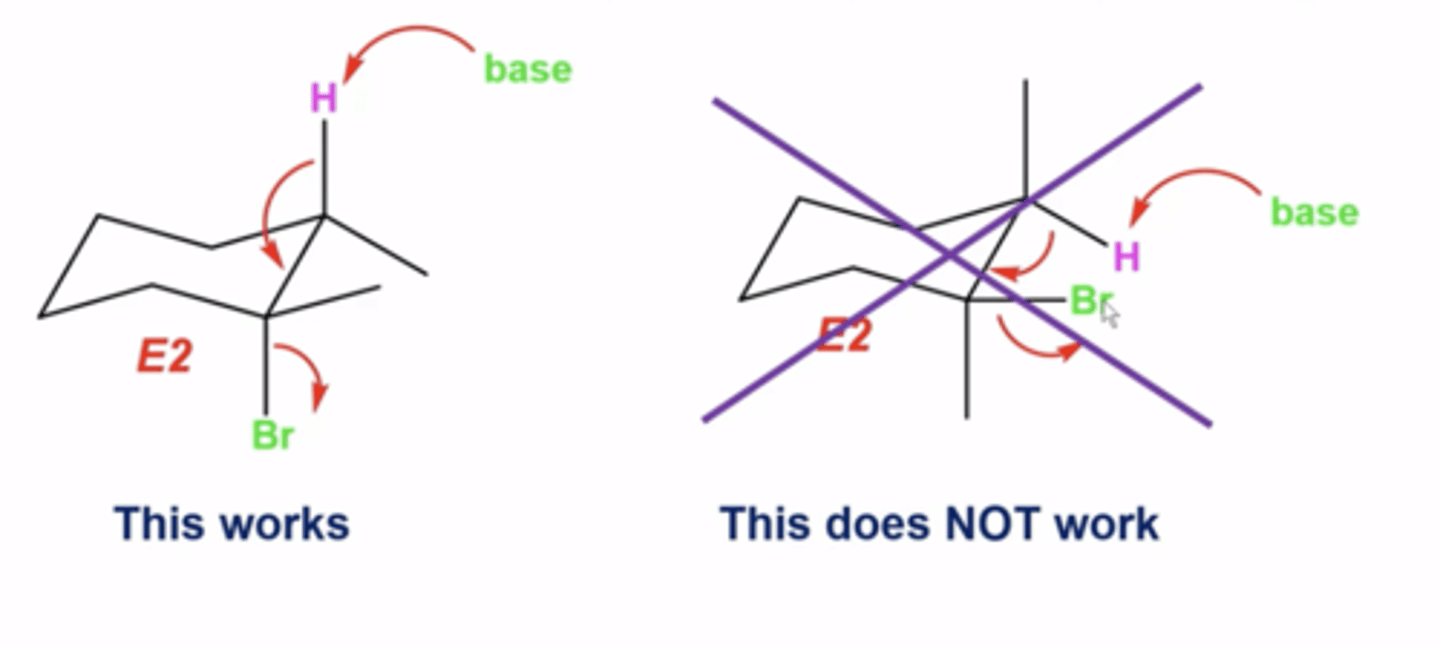
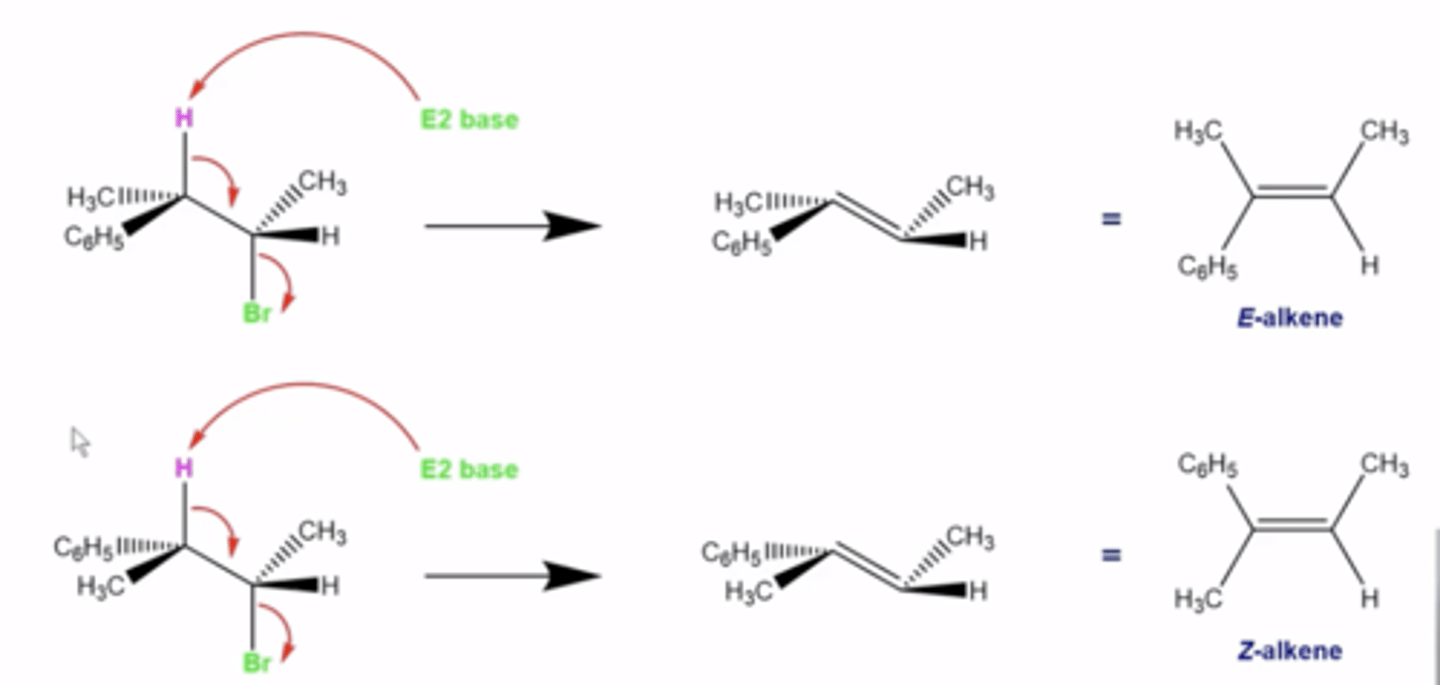
when will E2 reactions produce the Z alkene as the major product and the E alkene as the minor product?
-the eliminated H must be in the same three-dimensional plane, but pointing 180 degrees in the opposite direction as the L.G. (anti-periplanar)
-sometimes, depending on the configuration of the stereo centers and because H and the L.G. must be antiperiplanar, you get the Z-alkene product instead
during E2 reactions with rings, what must the orientation be for the attacked H and the L.G.?
they must be anti-periplanar, meaning that they must both be axial (which sometimes necessitates a ring-flip where you switch all axials and all equatorials)