Chemistry - Unit 5 Flashcards
5.0(2)
5.0(2)
Card Sorting
1/30
Earn XP
Description and Tags
Last updated 11:01 PM on 11/15/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
1
New cards
round 5 of chem flashcards, we got this u guys :D
2
New cards
this unit is chucking a bunch of stuff at us, so I'm going to put some review cards so we don't forget some important lil details
yeah.
3
New cards
aight let's start where this unit started, what is an element?
a substance made from 1 type of atom
4
New cards
monoatomic
made of 1 atom
5
New cards
diatomic
made up of 2 atoms
6
New cards
polyatomic
made of many, many atoms
7
New cards
compound
2 or more elements that are chemically bonded
8
New cards
molecule
group of atoms that are ~covalently~ bonded
9
New cards
what kind of atom cannot be in a molecule?
a metal
reminder: covalent bonding is only nonmetals. no forgetsies.
reminder: covalent bonding is only nonmetals. no forgetsies.
10
New cards
pOp QuiZ!
muahahaha
11
New cards
what do the little numbers in a chemical formula represent?
the amount of atoms of a certain element in a particle
12
New cards
what do the BIG numbers in a chemical formula represent?
the number of particles - also called a ~coefficient~
13
New cards
what do the letters in a chemical formula represent
the type of element
14
New cards
time for ur favvvvvv parttttt ->
~CHEMICAL REACTIONS~
15
New cards
WHAT IS COMBINATION/SYNTHESIS?
simple compounds -> complex ones
A + B -> AB
A + B -> AB
16
New cards
decomposition
~break it down~
complex compounds -> simple compounds
AB -> A + B
complex compounds -> simple compounds
AB -> A + B
17
New cards
single replacement
an atom tries to steal another atom's girl
or if we wanna be profesh, one atom takes the place of another
AX + B -> BX +A
or if we wanna be profesh, one atom takes the place of another
AX + B -> BX +A
18
New cards
BUT WAIT, what's special ab single replacement?
reaction only occurs if B is MORE REACTIVE than A
CHECK REACTIVITY. CHECK IT. IF NOT, I WILL SUE.
CHECK REACTIVITY. CHECK IT. IF NOT, I WILL SUE.
19
New cards
double replacement
two atoms switchie places
AX + BY -> AY + BX
write the POSITIVE ION FIRST
AX + BY -> AY + BX
write the POSITIVE ION FIRST
20
New cards
combustion
hydrocarbon reacts w/ oxygen and goes boom, becoming carbon dioxide and agua
or hydrocarbon reacts with oxygen gas in an ~exothermic~ reaction
or hydrocarbon reacts with oxygen gas in an ~exothermic~ reaction
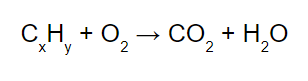
21
New cards
time fo' special weshal decomposition
yeehaw
22
New cards
special decomposition - chlorate
Metal (A)Chlorate(B) → Metal (A) Chloride + O2
occurs if there is a metal and chlorate in the reactant of a chemical equation
occurs if there is a metal and chlorate in the reactant of a chemical equation
23
New cards
special decomposition - hydroxide
element hydroxide -> element oxide + water
- only applies if singular bond ends w/ hydroxide
- only applies if singular bond ends w/ hydroxide
24
New cards
special decomposition - carbonate
XCO3 -> XO + CO2
- ionic compounds
- ionic compounds
25
New cards
what is the Law of Conservation of Matter/Mass?
MATTER CANNOT BE CREATED OR GO BOOM AND BYE (be destroyed), IT CAN ONLY CHANGE ITS FORM
26
New cards
OKAY there's some elements that are a little funky and have 2 or more atoms when by themself, what are they?
Sir S.P. BrINClHOF
(Sulfur 8, Phosphorous 4, Bromine 2, Iodine 2, Nitrogen 2, Chlorine 2, Hydrogen 2, Oxygen 2, Fluorine 2)
(Sulfur 8, Phosphorous 4, Bromine 2, Iodine 2, Nitrogen 2, Chlorine 2, Hydrogen 2, Oxygen 2, Fluorine 2)
27
New cards
predict the products then write and balance the following equations
mhm
28
New cards
calcium carbonate
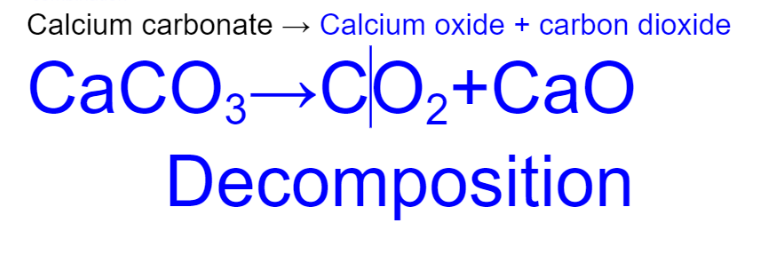
29
New cards
combustion of C12H26 (the numbers are supposed to be subscripts)
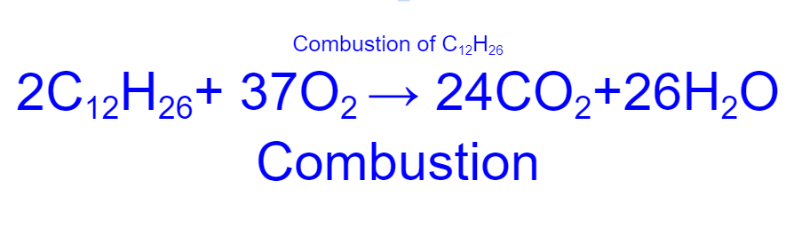
30
New cards
Iron (II) Chloride + Sodium Phosphate ->
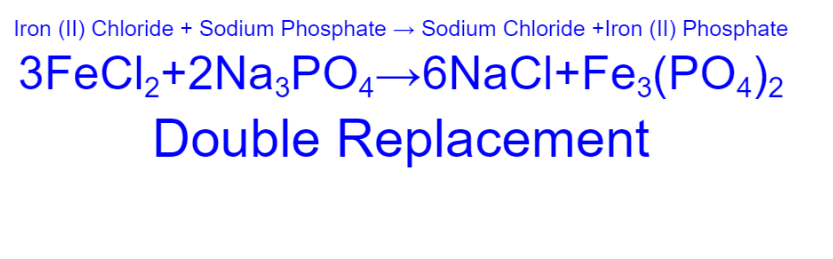
31
New cards
congrats ur at the end, check the resource page for practice :)