atoms, elements and compounds
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
23 Terms
what is the difference between a chemical and physical change?
physical is when the substance changes its physical state
eg: ice to water
chemical is when a new substance is formed during a chemical reaction
describe elements
any substance that can’t be broken down into a simpler one
only made up of one type of atom
what are the properties of metals?
usually solid at room temp
malleable and ductile
usually high melting and boiling points
good conductivity of heat and electricity
what are the properties of non metals?
solid, liquid or gas at room temp
usually soft or brittle
usually low melting and boiling point
very poor conductivity of heat and electricity
describe compounds
they’re pure substances which have two or more elements
eg; H2o or water
what is the difference between mixtures and compounds?
substances in a mixture have not had a chemical reaction
may be easy to separate them physically
compounds have had a chemical reaction
the properties are very different to the components
what is an exothermic reaction?
when heat energy is given out during a reaction
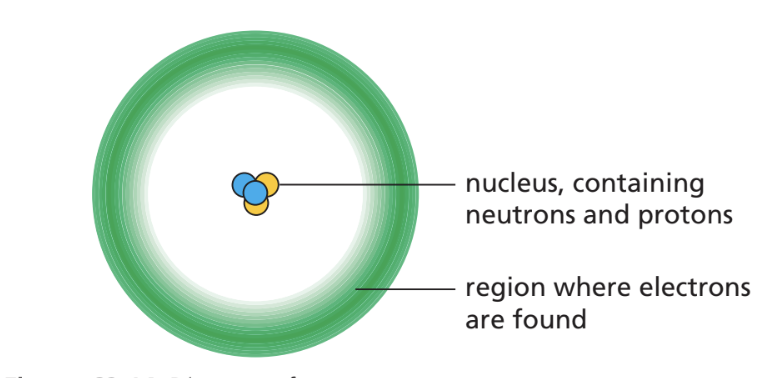
what is found in atoms?
protons (+ charge) and neutrons (no charge) which are in the nucleus
electrons (- charge) surround the nucleus
what is the atomic number equal to?
the number of protons in an atom
what is the nucleon number?
the total number of protons and neutrons
describe isotopes
they’re atoms of the same element which have a different number of neutrons but the same number of protons
what is electronic configuration?
2,8,8
the outer electrons are mainly responsible for an elements chemical properties
so elements in the same groups have similar ones
why do outer shell electrons become easier to lose?
because they get farther away from the nucleus
so there is less attraction between the nucleus and the outer shell electrons
because of the increased distance
what are ions?
electrically charged particles
an atom becomes an ion when is loses or gains electrons
describe ionic bonding
compounds with metal and non metals
electrons are transferred from metal to non metal atoms when this bond is formed
the atoms become more stable by getting full outer energy levels
what are the properties of ionic structures?
usually solids at room temp
have high melting and boiling points due to the strong electrostatic forces
ions are packed together in a lattice (regular arrangement)
cannot conduct electricity when solid because ions aren’t free to move
only in molten state
describe covalent bonding
compounds with non metals
shares electrons
what are the properties of covalent compounds?
simple molecular substances, usually gases liquids or solids
low melting and boiling point bc of the weak intermolecular forces of attraction
generally don’t conduct electricity when molten bc they don’t have ions
what is an allotrope?
when an element can exist in more than one physical form
what are the allotropes of carbon?
graphite, diamond
what are the properties of graphite?
has 3 covalent bonds
has a delocalised electron which allow graphite to conduct electricity
shiny
conducts electricity
soft material
uses: pencil, lube
what are the properties of diamond?
no delocalised electrons to enable diamond to conduct electricity
colourless
doesn’t conduct electricity
very hard
uses: jewellery, polishers
what are the use of metals?
electrical cables bc it has good electrical conductivity
making cars, bikes etc