AP BIO 1.1 - 1.3
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Why is water polar?
electronegativity of O causing slight negative charge of O and partial positive charge of H
Capillary action
spontaneous upward movement of liquid in a narrow tube or porous material, against gravity, caused by the adhesive forces between the liquid and the material, and cohesive forces within the liquid
What are the properites of water?
Dense solid state, high heat capacity, high heat of vaporization, universal solvent, cohesion+adhesion, surface tension
Surface tension
caused by hydrogen bonding, when molecules on the surface have nothing to pull them from the air above, so they form bonds with their neighboring molecule, causing stronger IMF.
gets tighter
more attracted to each other
stronger cohesion
Solvent
Because of its polarity, the negative ends attaches to solute cation and positive ends attract to solute’s anion.
it can dissolve anything with charge (polar/ionic)
Ice
the molecules of water gets pushed away from each other in solid state. As a result, the molecules of water are less dense and floats.
High Heat capacity
high amount of heat needed to raise the amount of heat
minimizes change in temperature
because of hydrogen bonds, water requires a large amount of energy to break before the water molecules can move faster and increase in temperature
High heat of vaporization
the hydrogen are very strong, so to break them requires a lot of energy. When the bonds are broken and H2O turns to it’s gas stage, the kinetic energy is lost, taking away energy, leaving a cooling effect
What is Carbon?
Key component for macromolecules
carbon bonds are exceptionally stableCarbon due to carbon's unique ability to form strong, covalent bonds with itself and other elements
Forms 4 bonds, so it serves as a backbone for macromolecules
Hydrocarbon
Covalent bonds between atoms in hydrocarbons store energy, making it a good fuel when burned
Can form 5 and 6 membered rings
Single or double bonds may connect the carbons in the ring
Nitrogen may be substituted for carbon
Benzene
An important hydrocarbon rings, used in some amino acids, cholesterol, and its derivatives
Isomers
molecules that have the same chemical formula but differ in placement/arrangement of atoms or types of bonds between atoms
What are the 3 types of isomers?
1. Structural isomers - have a different covalent arrangement of atoms.
2. Geometric isomers - have a different arrangement of atoms around a double bond
3. Enantiomers - molecules that share chemical formula and bonds but differ in 3D placement of atoms; mirror images
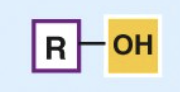
Hydroxyl group
polar
alcohols
increases the solubility and boiling points of molecules it's attached to due to H-Bonds
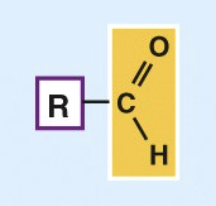
Aldehyde
c=o is very reactive
important in building molecules + energy-releasing reactions
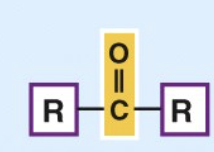
Keto
important in carbohydrates and in energy reactions
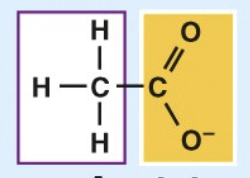
Carboxyl
acidic
carboxylic acids
Ionizes in living tissues to form COO⁻ and H⁺
Enters into condensation reactions by giving up its —OH group.
Some important in energy-releasing reactions.
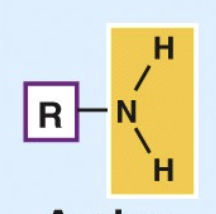
Amino
Basic
Accepts H⁺ in living tissues to form —NH₃⁺
Enters into condensation reactions by giving up H⁺.
Phosphate
Negatively charged
Enters into condensation reactions by giving up —OH
When bonded to another phosphate, hydrolysis releases much energy
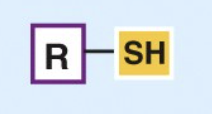
Sulfhydryl
By giving up H, two —SH groups can react to form a disulfide bridge (S—S), stabilizing protein structure
What are the 4 types of macromolecules?
carbohydrates, lipids, proteins, nucleic acid
monomers and polymers
Macromolecules consist of individual subunits called monomers
Monomers are linked together via covalent bonds into polymers
Dehydration synthesis
joins monomors together
forms a covalent bond
releases H2O
releases energy
Hydrolysis
breaks polymers
1 monomer gain OH, 1 gains H
requires energy
Enzymes
speed u reaction for hydrolysis and dehydration synthesis
enzymes that helps with hydrolysis ends with -ase
Anabolism
link simpler molecules to form more complex ones, requires energy that are captured in chemical bonds that form
Catabolism
break down molecules, releasing energy
metabolism
chemical reactions in a living organism that sustain life, transforming food into energy, providing building blocks for cells and tissues, and eliminating waste products
anabolism + metabolism
creates more disorder
Entropy
If there are fewer products than reactants, the disorder is reduced; this requires energy to achieve
If a chemical reaction increases entropy, its products are more disordered or random than its reactants.
+G(energy to do work) → -S (entropy)
Laws of thermodynamics
Energy is neither created nor destroyed
Second law: Disorder (entropy) tends to increase.
When energy is converted from one form to another, some of that energy becomes unavailable for doing work (is lost from the system often as heat energy).
In a closed system with repeated transformation, free energy decreases and unusable energy increases