Organic Chem Ch. 5: Sterioisomers
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
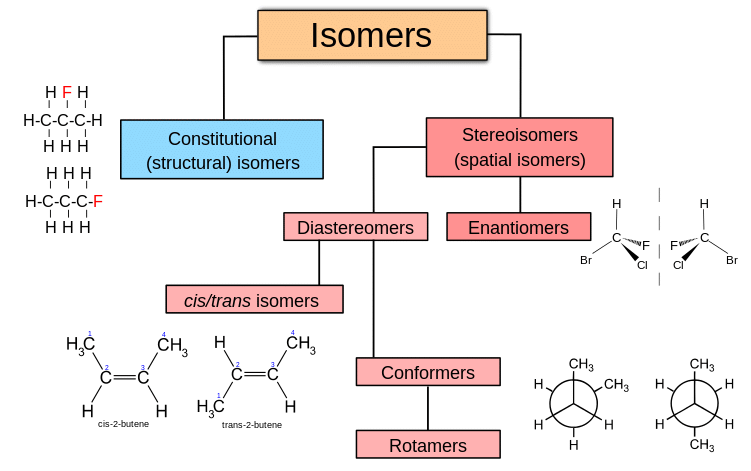
Constitutional Isomers
Have the same molecular formula but differ in their connectivity of atoms
Stereoisomers
Have the same connectivity of atoms but differ in their spatial arrangement. The terms cis and trans are used to differentiate stereoisomeric alkenes as well as disubstituted cycloalkanes
What are chiral “hand” objects?
An object that cannot be superimposed on its mirror image. Meaning it has a distinct left and right. Cannot align no matter how it is rotated
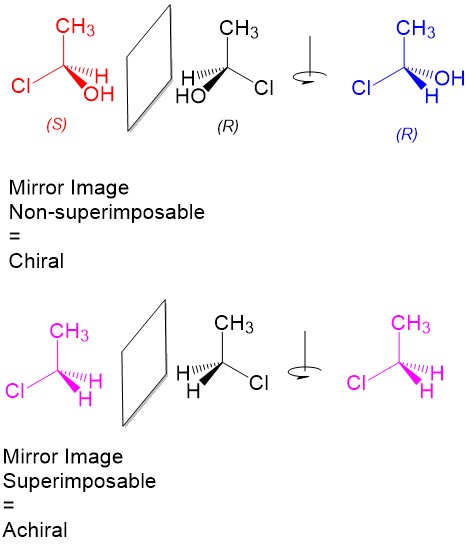
Superimposable
Two objects can be placed directly on top of each other, aligning perfectly
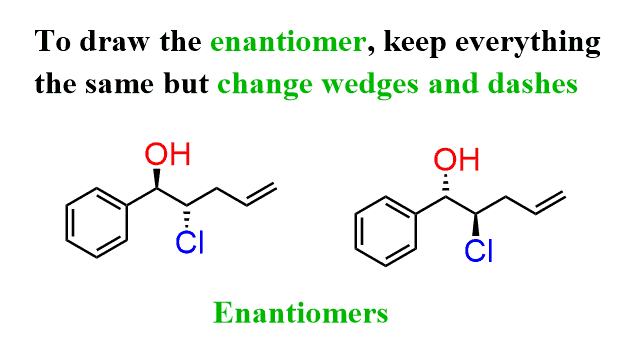
Enantiomer
A compound with one chiral center will have one nonsuperimposable mirror image. Same connectivity but different 3-D shapes
What is the Chan-Ingold-Prelog system used for?
To assign the configuration of a chiral center
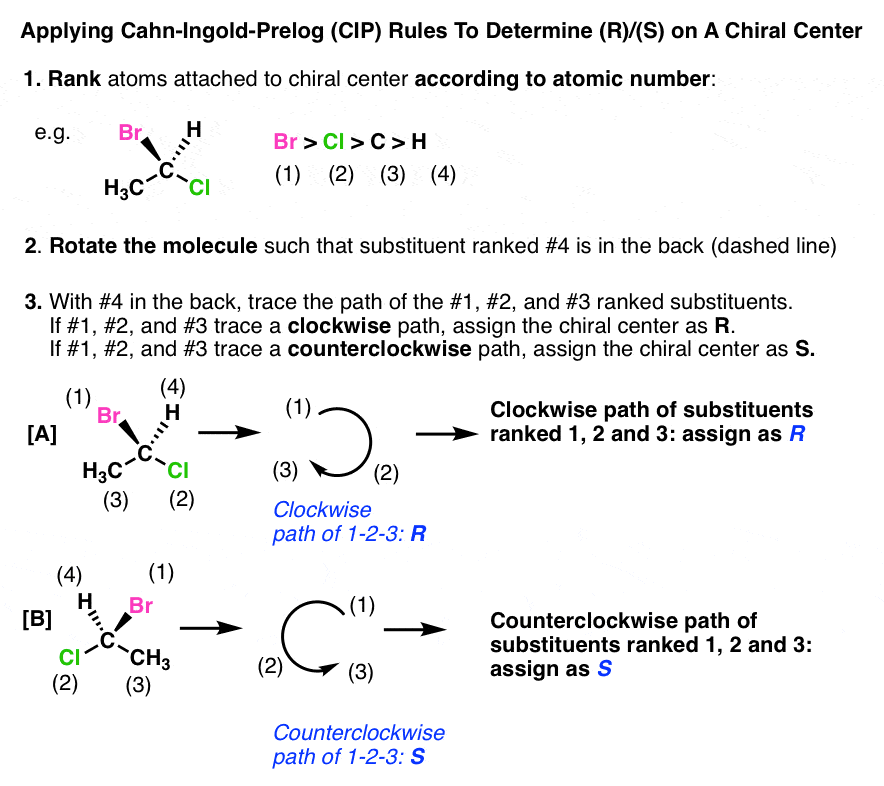
Which way does R go?
Clockwise
Which way does S go?
Counterclockwise
What is a polarimeter?
This is a device used to measure the ability of chiral organic compounds to rotate the plane of plane-polarized light
What are compound that are optically inactive?
Compounds that do not rotate plane-polarized light
Compounds that rotate the plane of plane-polarized light are said to be:
Optically active
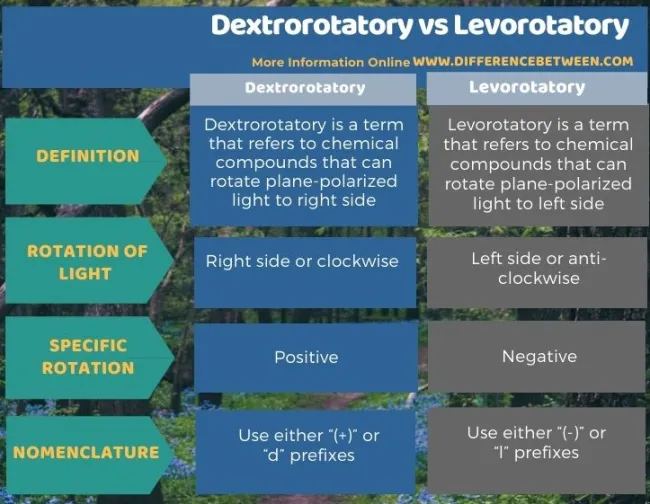
Compounds that have a positive rotation (+) are said to be
Dextrorotatory
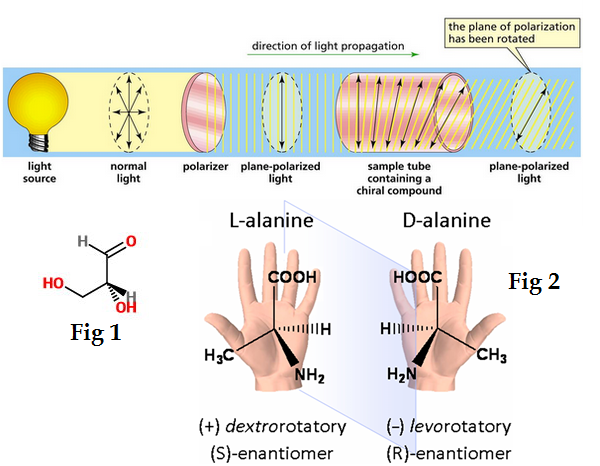
Compounds that exhibit a negative rotation (-) are said to be:
Levorotatory
Optically pure
A solution containing a single enantiomer
Racemic mixture
A solution containing equal amounts of both enantiomers
Enantiomeric excess
A solution containing a pair of enantiomers in unequal amounts
The number of stereoisomers of a compound can be no larger than _____, where n= the number of chiral centers
2^n
What type of stereoisomers are mirror images?
Enantiomers are mirror images
What type of stereoisomers are not mirror images?
Diastereomers are not mirror images
What are the two different kinds of symmetry?
Rotational symmetry
Reflectional symmetry
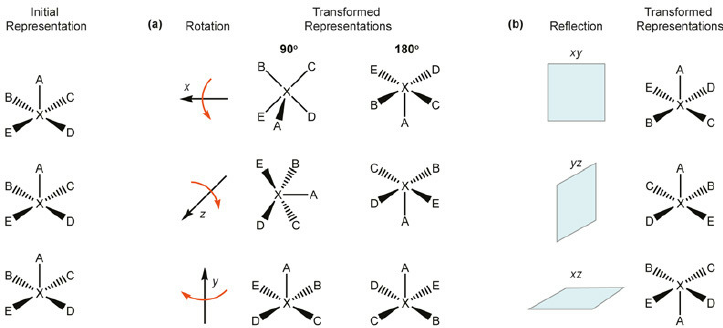
A compound that possesses a _________ will be achiral
Plane of symmetry
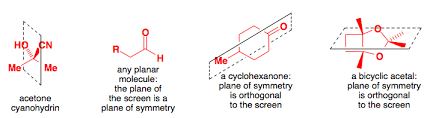
A compound that lacks a plane of symmetry will most likely be:
Chiral typically lack a plane of symmetry
Meso compound
A meso compound contains multiple chiral centers but is achiral because it possesses reflectional symmetry. A family of stereoisomers contains a meso compound will have fewer than 2n sterioisomers
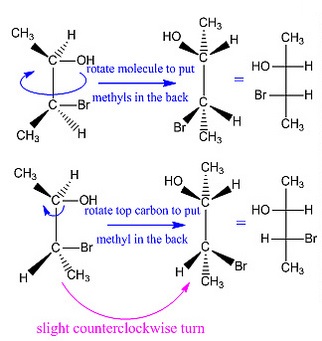
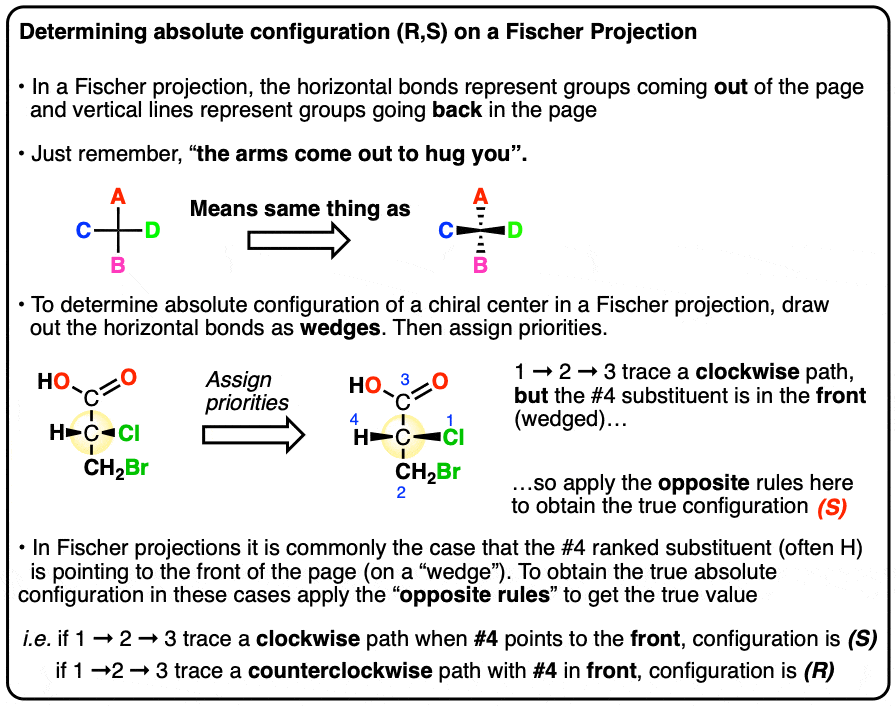
What are Fischer projections?
These are drawings that convey the configurations of chiral centers, without the use of wedges and dashes. All horizontal lines are understood to be edges and all vertical lines are understood to be dashes
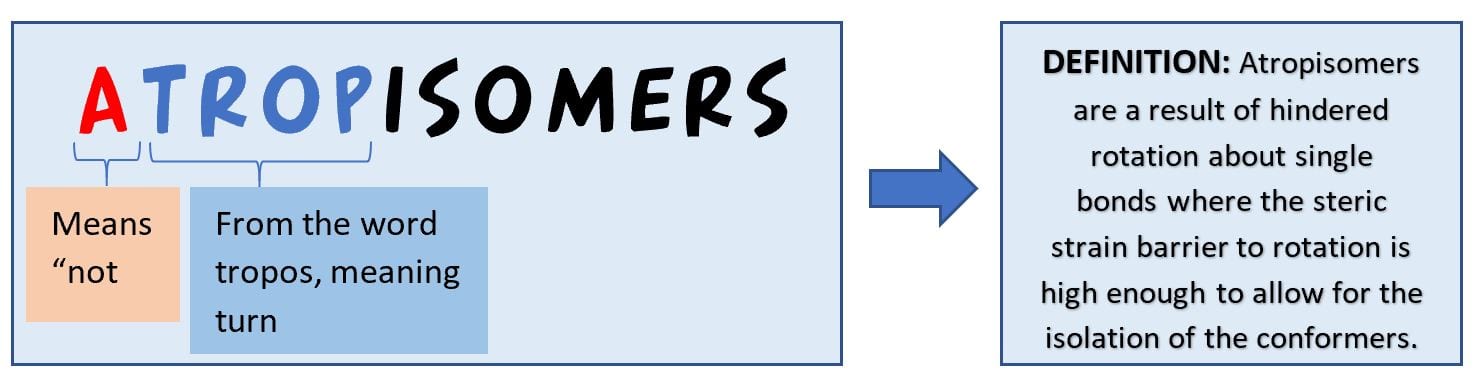
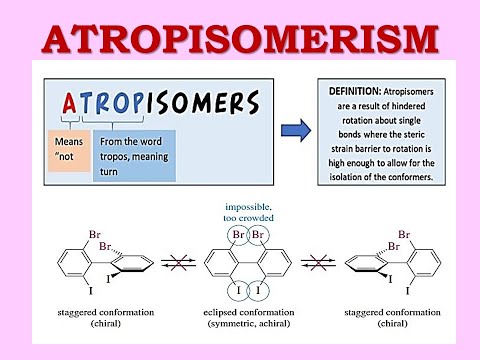
What are atropisomers?
These are a type of stereoisomers that result from the hindered rotation of a single bond
Allenes
These are a class of compounds that can be chiral despite the absence of a chiral center. They contain C=C bonds