Bio - Metabolism + Electron transport chain
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
Energy
the capacity to perform work (cause change)
Potential Energy
stored energy that has the potential to do work.
Chemical Energy
a form of potential energy. Energy stored in Chemical Bonds.
Kinetic Energy
energy in Motion.
Potential Energy - Example
A ball about to be dropped. Water behind a dam.
Chemical Energy - Example
Bonds in glucose molecules.
Kinetic Energy - Example
A dam opening to let water fall. Membrane protein opening to let ions on the inside of the cell out.
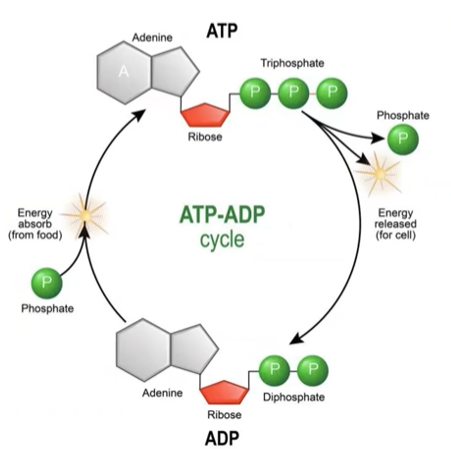
Adenosine Triphosphate (ATP)
“Energy currency”. Has high potential energy. Phosphate groups attached by high energy bond. Releases energy when bond breaks to run cellular processes. Unstable.
Adenosine Diphosphate (ADP)
What is left after a phosphate is broken off of ATP. Low potential energy.
Thermodynamics
The study of energy transformation
1st Law of Thermodynamics
Energy cannot be created nor destroyed. Can be converted, transformed, transferred between different forms
2nd Law of Thermodynamics
Energy conversion is inefficient. Every time we convert energy, we "Lose" some as heat. (why we get hot and need water in our body)
Energy Cycling
Review - Energy Cycling
Mechanical Work
This is when cells move things.
Ex. Cilia on some cells wave back and forth to move fluid.
Transport Work
This is when cells move molecules against the concentration gradient.
Ex. Active transport (Bulk transport…)
Chemical Work
Endergonic reaction (reaction the requires Energy)
Energy Coupling
Running two reactions at the same time (releasing energy and taking energy). Cells are Multitasking.
Catabolic Pathway
Breaking a molecule down (release energy).
Ex. Cellular respiration
Anabolic Pathway
Builds molecules (stores energy).
Ex. Building protein from amino acids.
Metabolism
Is a series of chemical reactions. Many require energy (activation energy) some are reliant on Enzymes (which lower cost of energy)
Oxidation - Reduction reactions
Transfer of electron(s) from one Reactant to another.
Reduction
Is the Gain of electron(s).
Oxidation
Is the Loss of electron(s).
Ex. Glucose goes through oxidation in cellular respiration