specific heat capacity
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What is specific heat capacity
The amount of energy needed to raise the temp of 1 kg of a substance by 1 degrees C
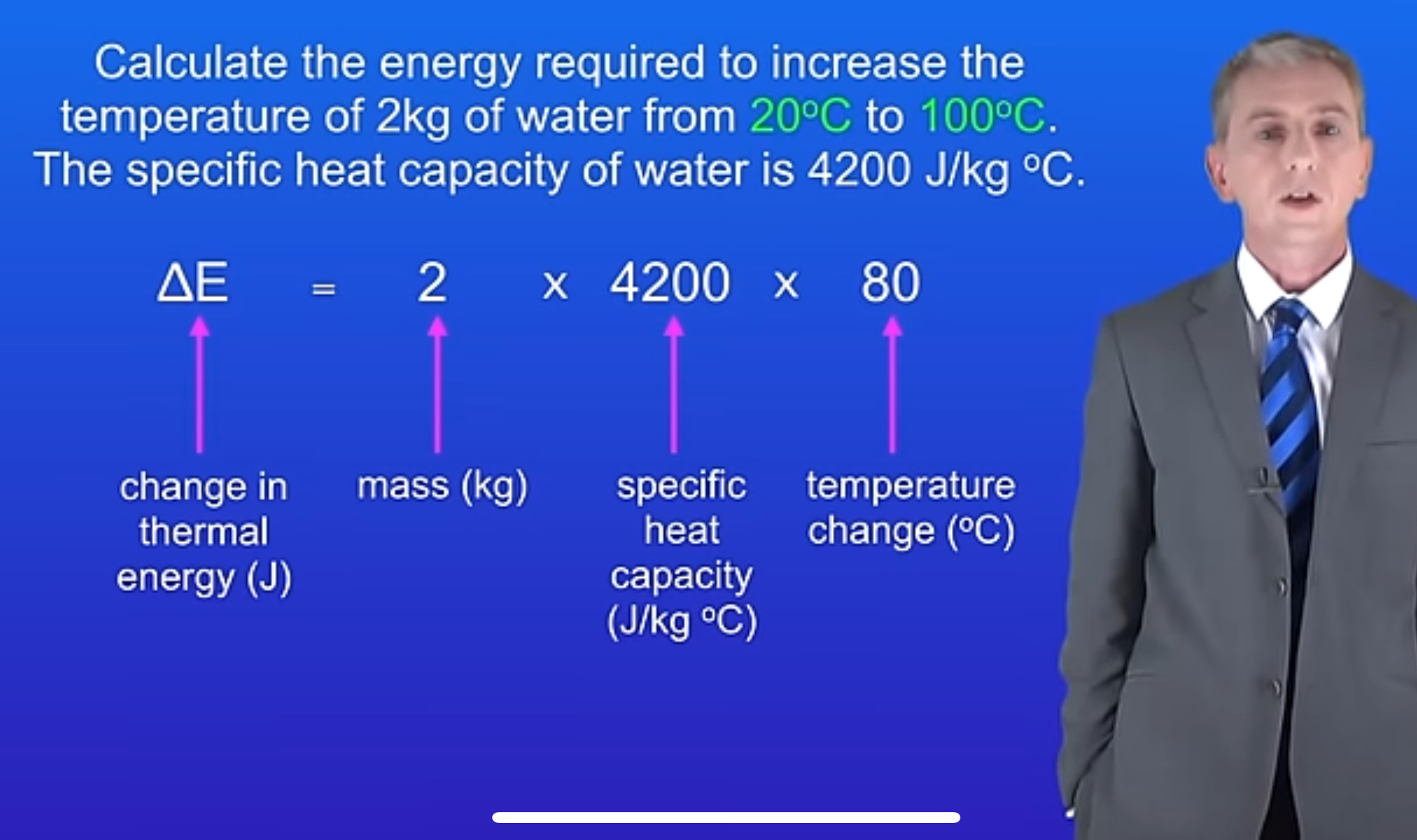
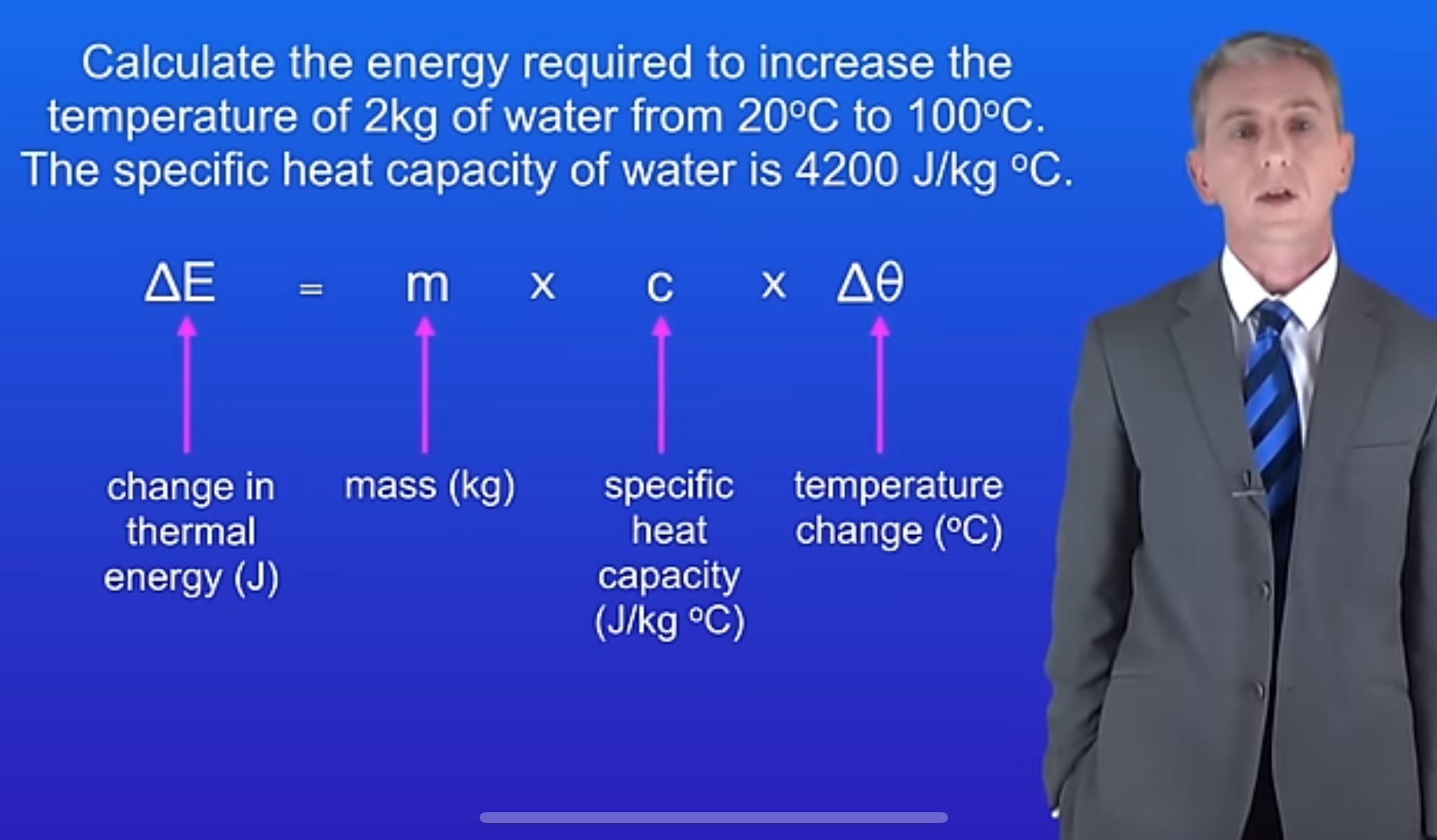
What is the formula for energy change using specific heat capacity?
ΔE = mcΔθ
In the formula ΔE = mcΔθ, what does 'm' stand for?
Mass, measured in kilograms (kg).
What is the unit of specific heat capacity?
Joules per kilogram per degree Celsius (J/kg°C).
Which has a higher specific heat capacity: water or metals?
Water.
Why does water heat up more slowly than metals?
Because it has a higher specific heat capacity, so it requires more energy to raise its temperature.
What equipment is used to investigate the specific heat capacity of a block?
An immersion heater, power supply, thermometer, and insulation.
Why is the block wrapped in insulation during a specific heat capacity experiment?
To reduce heat loss to the surroundings.
How do you calculate the energy supplied in a specific heat capacity experiment?
E = IVt (where I = current, V = voltage, and t = time).
How can you improve accuracy in specific heat capacity experiments?
Use thermal insulation, measure the initial temperature accurately, and ensure good thermal contact (e.g., with water or thermal paste).
Also, if the hole in the in your material is bigger than your The thermometer you could put a small amount of water in the hole with the thermometer. This helps the thermometer to measure the temperature of the block more accurately as water is a better thermal conductor than air
What is the specific heat capacity practical for solid material
Measure the mass of the block using a balance.
Wrap the block in insulation to reduce heat loss. Insert a thermometer and an immersion heater into the holes of the block.
Record the initial temperature of the block.
Switch on the power supply to the heater and start a stopwatch.
Record the current (I) and voltage (V) of the power supply.
Keep the heater on for a measured time (t).
Measure the final temperature of the block.
Calculate the energy supplied to the heater using:
Repeat this experiment with different materials to see how their heat capacities compare
Extra note how to investigate specific heat capacity of liquid
Just place the heater and thermometer in an insulated beaker filled with a known mass of the liquid and follow same steps
Practice questions