Aerobic Respiration II
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
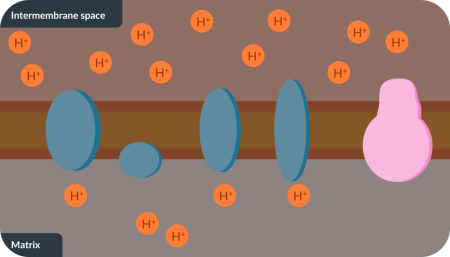
Protons move down the proton gradient, from the…………….. …… to the ……..
inter-membrane space
matrix
Which of the following is an example of chemiosmosis?
Protons actively pumped across the thylakoid membrane into the thylakoid lumen.
Sodium ions diffusing across the cell membrane from the extracellular space to the cytoplasm.
Potassium and sodium ions actively transported into a neurone’s axon.
B
as ions move from are of high concnenration to area of lower concnetration across a partially permebale membrane
To produce ATP in a final stage of aerobic respiration, an enzyme is needed.
This enzyme is called…
ATP synthase.
To catalyse the production of ATP, ATP synthase needs energy.
This energy is supplied by…
diffusion of protons down a proton gradient.
diffusion of electrons down an electron gradient.
diffusion of protons against a proton gradient.
diffusion of electrons against an electron gradient.
A
The movement of ions across a partially permeable membrane, from an area of a high concentration to an area of low concentration is known as…
ion diffusion.
chemiosmosis.
ion osmosis.
chemidiffusion.
B
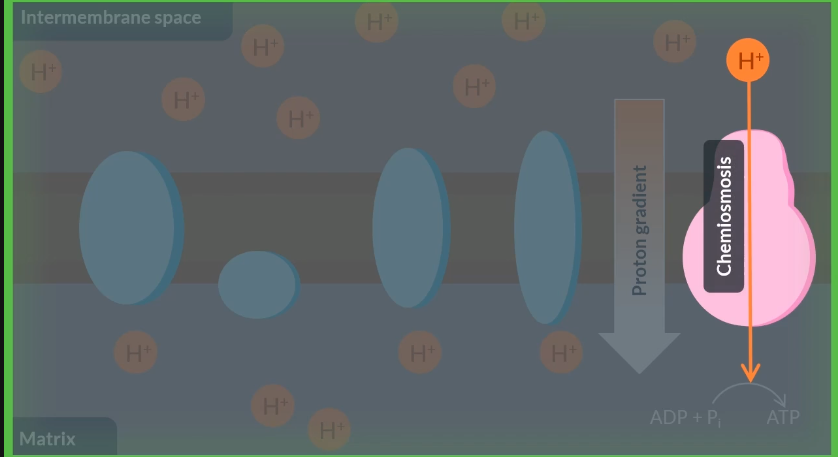
To produce ATP in the final stage of aerobic respiration, an enzyme called ……… ……….is used.
To catalyse the production of ATP by this enzyme, energy is supplied through the diffusion of……….down a………..gradient. As a result, these particles move from the………. …….to the matrix.
Finally, the movement of ions across a partially permeable membrane, from an area of a high concentration to an area of low concentration, is known as……………
atp synthase
protons
proton
intermebrane space
chemiomosis
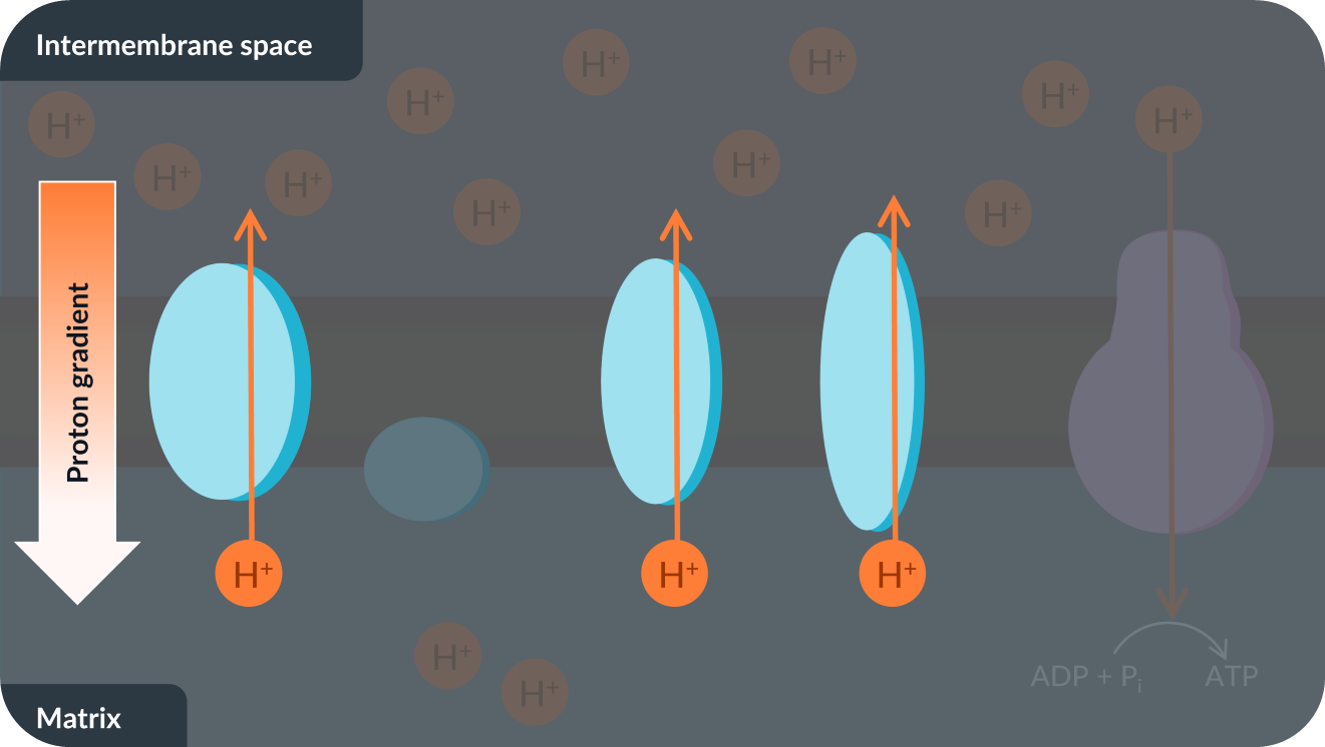
We’ve seen that the energy ATP synthase needed to catalyse the production of ATP is provided by chemiosmosis: the diffusion of protons down the proton gradient through ATP synthase.
In order to make sure that ATP synthase has enough energy to keep producing ATP, the proton gradient needs to be maintained.
To maintain the proton gradient, protons are actively transported from the matrix to the intermembrane space. This ensures that there’s always a higher concentration of protons in the intermembrane space compared to the matrix.
Finally, the active transport of protons into the intermembrane space also requires energy.
The source of this energy is a bit strange…
To understand where the energy comes from, we'll first need to take a look at oxidation-reduction reactions and electrons, next!
In mitochondria, there is a higher concentration of protons in the…. ……..compared to the…….
To maintain this proton gradient, proteins move the protons by the process of …….. ………..
intermembrane space
matrix
active transport
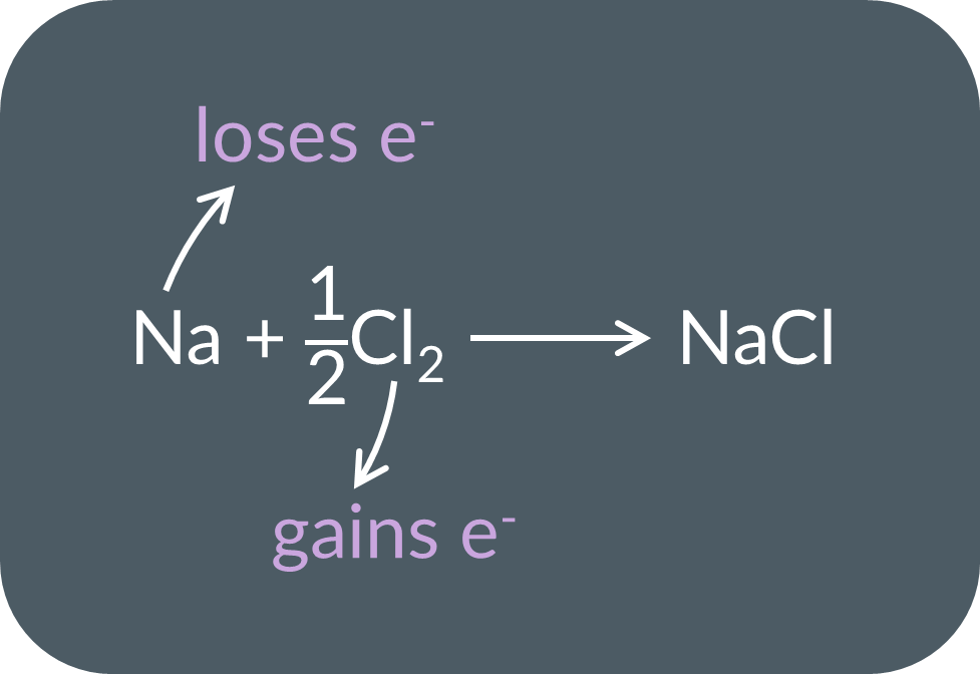

Here is a reaction:
Ca2++2e−→Ca
K→K++e−
Li++e−→Li
Be→Be2++2e−
This is an example of a(n)…
oxidation reaction.
reduction reaction.
reduction
oxidation
reduction
oxidation
Proteins in the inner membrane need energy to actively transport protons across the membrane.
To generate this energy, a molecule donates…
protons to the first membrane protein.
electrons to the first membrane protein.
hydrogen ions to the first membrane protein.
B
After a molecule has donated electrons to the first membrane protein, the electrons move through other proteins in the membrane.
These proteins are collectively known as the…
electron transfer chain.
ion transport chain.
proton transfer system.
protein transport system.
A
Electrons move through the electron transfer chain via a series of…
hydration-hydrolysis reactions.
addition-subtraction reactions.
oxidation-reduction reactions.
C
If the electron transport chain grinds to a halt, this means that protons cannot…
diffuse into the intermembrane space.
be actively transported into the matrix.
be actively transported into the intermembrane space.
diffuse into the matrix.
C
If there isn’t a proton gradient, this means that…
ATP can’t be produced.
protons can’t be actively transported.
ATP synthase stops functioning.
A
Our bodies can only carry out aerobic respiration when there’s enough…
oxygen.
water.
ATP.
A
Our bodies need water to remain alive, but do not need water specifically for aerobic respiration.
Our bodies use aerobic respiration to produce ATP from ADP.
Electrons at the end of the transfer chain react with…
Select all that apply
carbon dioxide
protons
ions
oxygen
ADP
The electrons react with these to form………..
B D
water
Electrons at the end of the electron transfer chain react with oxygen and protons to form water. For this reason, oxygen is known as the…
final proton donor.
terminal proton acceptor.
terminal electron donor.
final electron acceptor.
D
Proteins in the inner membrane of a mitochondrion need energy to actively transport protons across the membrane.
To generate the energy, …………. move through these proteins via a series of………… …………….. reactions.
Collectively, these proteins in the inner membrane are known as the……. ………… ……..Finally, oxygen and protons react with……..to form………...
As a result, oxygen is known as the…… ……….. ………..
electrons
oxidation-reduction
electron transport chain
electrons
water
final electron acceptor
When electrons move through proteins on the inner membrane, what reactions occur to facilitate the exchange of electrons down the chain?
Oxidation-reduction
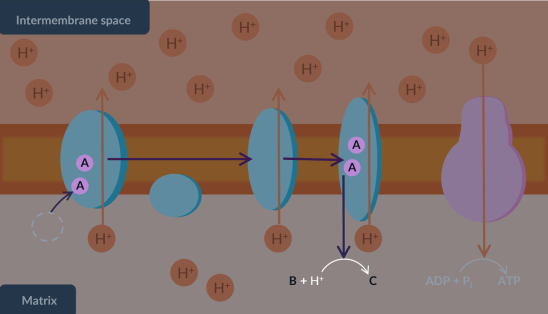
Complete the following diagram by identifying A,B and C.
B is also known as the…
electrons
oxygen
water
final electron acceptor
After it’s produced, NADH travels to the inner mitochondrial matrix and donates two electrons to the first protein in the electron transfer chain.
This means that NADH is…
dehydrogenated.
hydrolysed.
oxidised.
reduced.
C
As well as reduced NAD, the Krebs cycle also produces…
FADH2
NADH
ADP
NADP
A
The electrons in the electron transfer chain are donated by…
NADH
FADH2
NAD
FAD
NADP
NADPH
A B
whcih lose elctrons to become FAD and NAD
DOnation of electrons from NADH
We’ve previously seen that when NADH donates two electrons to the electron transfer chain, the reaction produces a proton and NAD.
However, this isn’t quite the case. The donation of two electrons from NADH actually forms a positively charged molecule of NAD, represented like this: NAD+.
And, it’s NAD+, not NAD, that reacts with hydrogen in glycolysis and the Krebs cycle!
However, to keep things simple, at A-level, NAD+ is referred to as NAD. Additionally, you won’t be expected to remember how many electrons NADH, or FADH2, donates to the electron transfer chain!
In the inner mitochondrial membrane, reduced NAD and reduced FAD are oxidised. During this reaction, what do the reduced NAD and reduced FAD donate to the membrane proteins?
Electrons
Protons
Ions
Phosphate
A
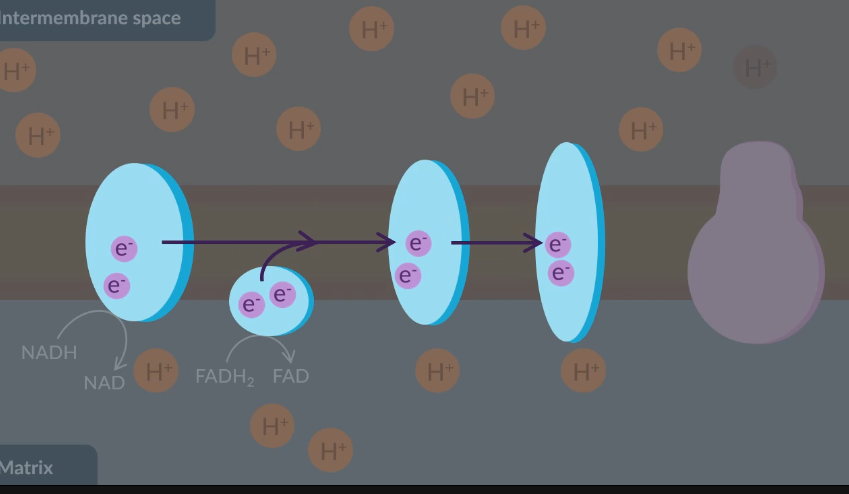
As they travel down the electron transfer chain, electrons…
release energy into the membrane.
absorb energy from the proteins.
exchange energy between themselves.
transfer energy to proteins.
D
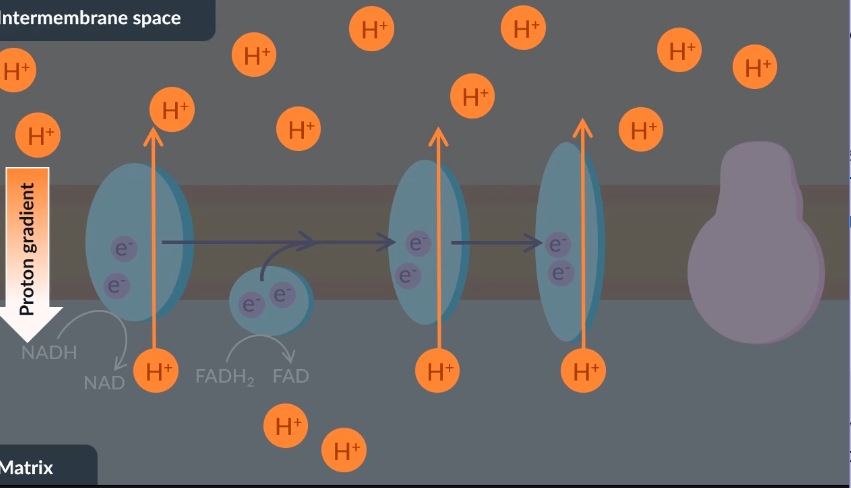
The energy that electrons transfer to membrane proteins is used to actively transport…
NAD.
protons.
NADH.
potassium ions
B so hat the proton grandient is maintained between the intermembrane space and th ematrix for diffusion down ATP synthase
The electrons at the end of the electron transfer chain react with…
Select all that apply
oxygen.
carbon dioxide.
water.
protons.
NAD.
FAD.
B D
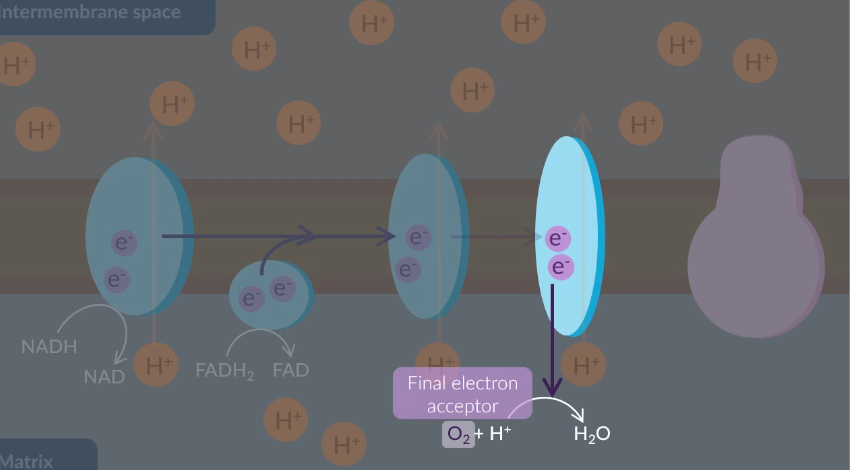
Protons in the intermembrane space of a mitochondrion diffuse down the proton gradient and back into the matrix via a protein. What is the name of this protein?
ATP synthase
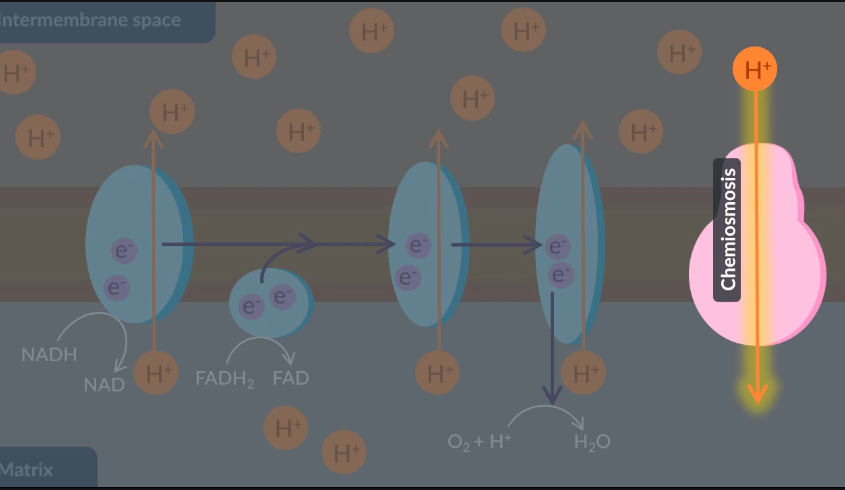
Although NADH and FADH2 are oxdised to form ……… …………. by providing electrons to elctron tranfer chain , it is the ………….. of ADP and ……. → ………. which gives this process the name of oxidative …………. which produced around ……… ATP and is the …….. and final stage of …….. repsiration
NAD
FAD
phosphotelation
Pi
ATP
phosphorelation
30
fourth
aerodbic
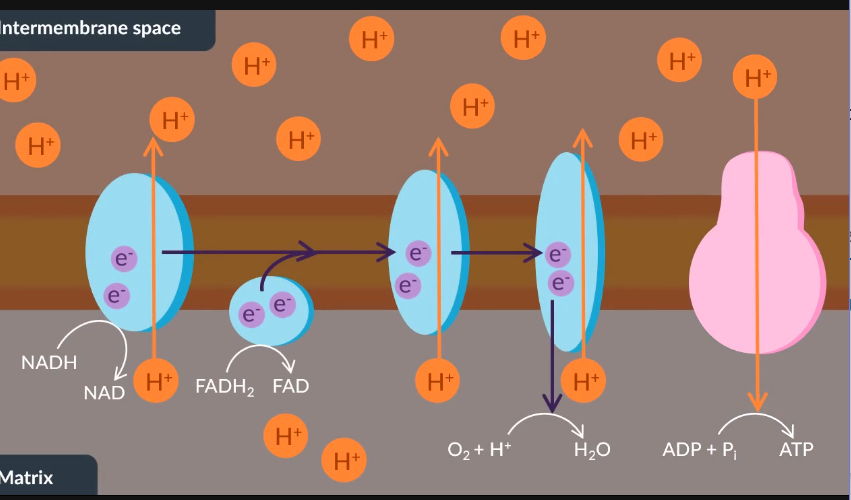
In oxidative phosphorylation, ………..supplied by……..and……are transferred through the electron transfer chain via a series of oxidation-reduction reactions.
This series of reactions provides………….to the electron transport chain. As a result,………….move from the matrix to the intermembrane space via………….transport.
This movement ensures that a………….gradient is maintained across the inner membrane.
Next, the electrons in the final protein react with oxygen and protons to form……….
In this instance, oxygen is also known as the …… ………. ……….. .
Finally, chemiosmosis through the enzyme called………..results in the production of ATP.
electorns
NADH
FADH2
energy
protons
active
proton
water
final electron acceptor
atp synthase
The transfer of electrons through the electron transfer chain…
provides energy to the inner membrane proteins.
oxidises the proteins.
allows protons to be transferred from the matrix to the intermembrane space.
allows protons to be transferred from the intermembrane space to the matrix
A C
The transfer of protons from the intermembrane space to the matrix happens via…
diffusion.
active transport.
chemiosmosis.
C they are transported thru ATP syntahse and teh movement of ions of charge are called cheismosi
Describe the stages of oxidative phosphorylation.
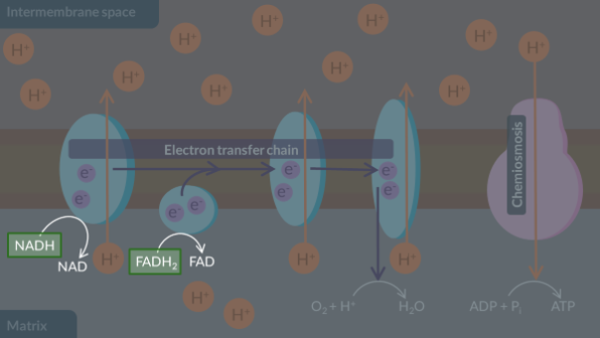
In oxidative phosphorylation, electrons supplied by reduced NAD and reduced FAD are transferred through the electron transfer chain via a series of oxidation-reduction reactions.
Next, these oxidation-reduction reactions provide energy to the electron transfer chain. As a result, protons move from the matrix to the intermembrane space via active transport. This ensures that a proton gradient is maintained across the inner membrane.
Additionally, the electrons in the final inner membrane protein react with oxygen and protons to form water. Through this reaction, oxygen is also known as the final electron acceptor.
Finally, chemiosmosis through ATP synthase results in the production of ATP.
Which process produces ATP?
Select all that apply
Substrate-level phosphorylation
Oxidative phosphorylation
A B
Which process requires the donation of a phosphate group from a substrate?
Select all that apply
Substrate-level phosphorylation
Oxidative phosphorylation
A
Which process takes place in the cytoplasm of a cell?
Select all that apply
Substrate-level phosphorylation
Oxidative phosphorylation
A (B is Takes place in the mitochondria)
How Mitochondria Are Adapted For Respiration
In AS biology, you may have looked at the structure of a mitochondrion.
Mitochondria have an inner mitochondrial membrane that folds to form cristae, which provide a large surface area for molecules involved in respiration.
Now, at A-level, we can say that this is specifically because oxidative phosphorylation takes place in the inner mitochondrial membrane: the cristae increase the surface area of the membrane, which in turn increases the space available for oxidative phosphorylation. This means that a lot of ATP can be made at one time.
Finally, the number of cristae can vary depending on the amount of ATP that a particular cell needs.
For example, heart cells need more ATP than liver cells. So, mitochondria in heart cells have a larger number of cristae than mitochondria in liver cells!
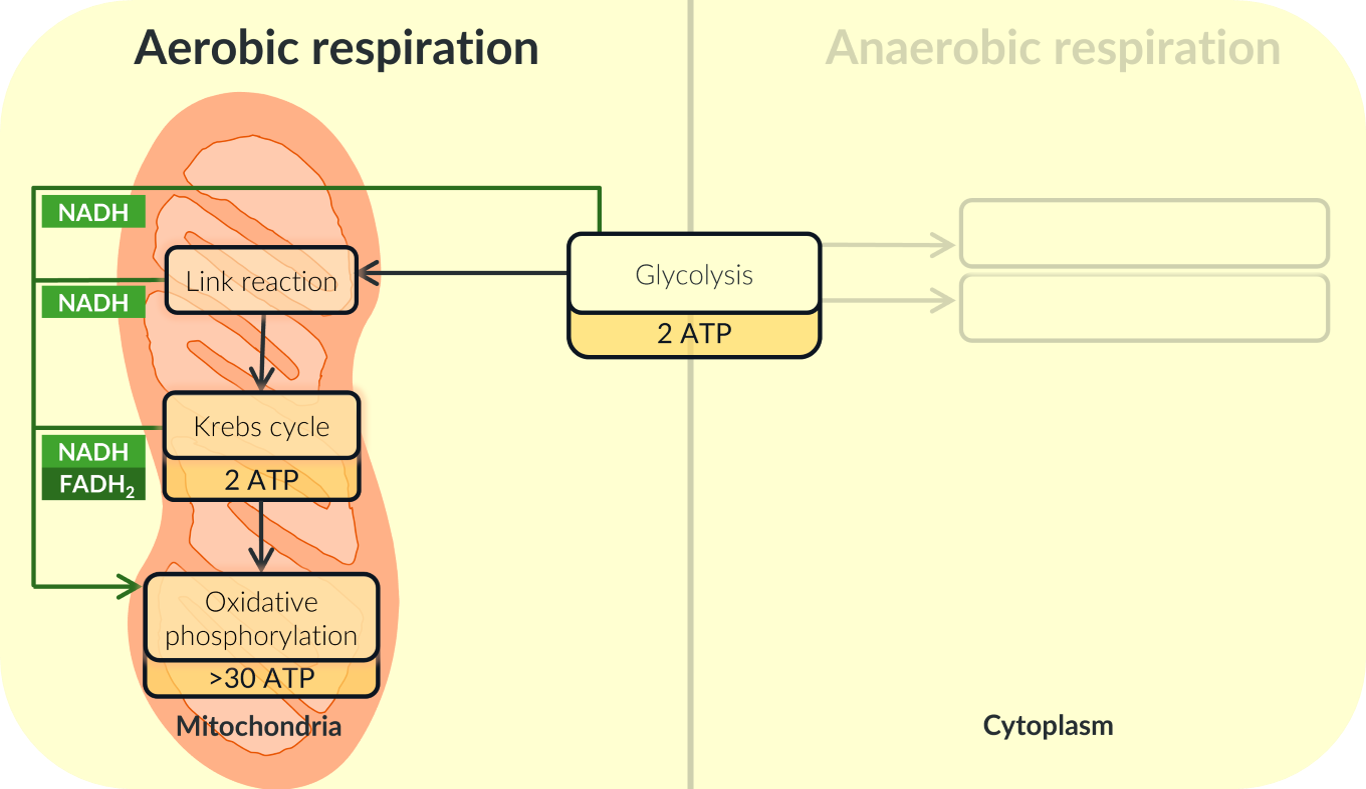
The Entirety of Aerobic Respiration
there are 4 stges of aerobic repsiration
Of these four stages, oxidative phosphorylation is the only stage to require NADH and FADH2. FADH2 is only produced in the Krebs cycle, while NADH is produced in glycolysis, the link reaction and the Krebs cycle.
We can represent this on our diagram showing the four stages of aerobic respiration like this:
We can also see how NADH and FADH2 are used in oxidative phosphorylation like this:
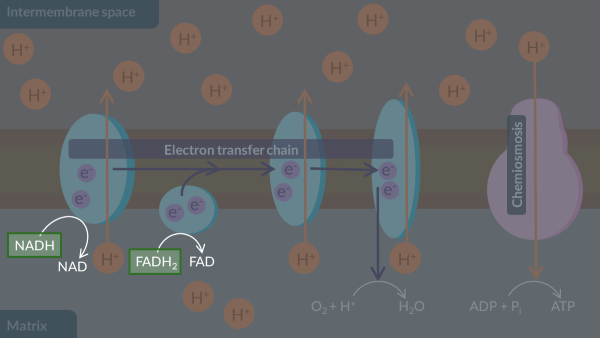
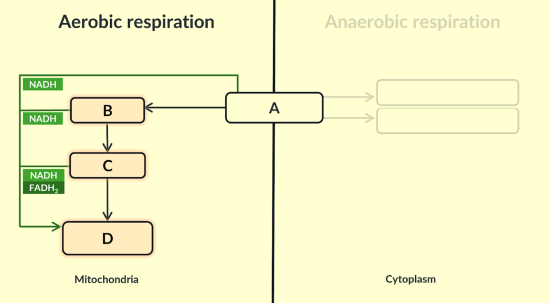
Here is an overview of aerobic respiration. Name each stage labelled A,B,C and D.
A: glycolysis
B: link
C: krebs
D: oxidative phosphorelation
During which stage(s) is NAD produced?
Glycolysis
Link reaction
Krebs cycle
Oxidative phosphorylation
D
During which stage(s) is NADH produced?
Select all that apply
Glycolysis
Link reaction
Krebs cycle
Oxidative phosphorylation
A B C
During which stage(s) is both NADH and FADH2 required?
Select all that apply
Glycolysis
Link reaction
Krebs cycle
Oxidative phosphorylation
D
During which stage(s) is a proton gradient required?
Select all that apply
Glycolysis
Link reaction
Krebs cycle
Oxidative phosphorylation
D
In which stage(s) of aerobic respiration is the inner mitochondrial membrane involved?
Select all that apply
Glycolysis
Link reaction
Krebs cycle
Oxidative phosphorylation
D
In which stage(s) of aerobic respiration is there a net yield of 2 ATP molecules?
Select all that apply
Glycolysis
Link reaction
Krebs cycle
Oxidative phosphorylation
Submit
A C
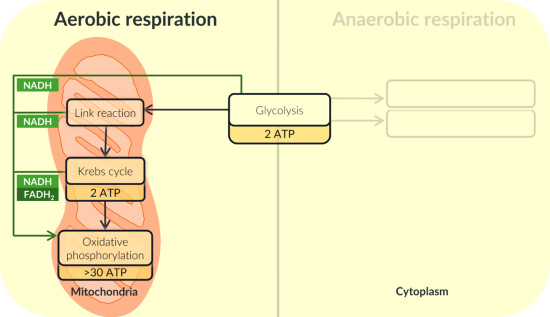
Describe the role of oxygen in aerobic respiration.
Protons produced in glycolysis and the Krebs cycle combine with oxygen and electrons to form water.
As a result, oxygen allows the electron transfer chain to function.
Due to its essential role in the electron transfer chain, oxygen is referred to as the final electron acceptor.
One type of mitochondrial dysfunction prevents the mitochondria cristae from being fully formed.
Suggest how a lack of mitochondrial cristae can affect respiration.
There is a smaller surface area of the inner mitochondrial membrane. This means that there are fewer electron transport chains for oxidative phosphorylation. As a result, less ATP is produced in aerobic respiration / oxidative phosphorylation.