chp 19 the tricarboxylic acid cycle
1/114
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
115 Terms
the citric acid cycle is the central pathway for
oxidizing all metabolic fuels
most of the energy yield from the oxidation of substrates in the TCA is stored in what
reduced electron carriers (NADH and FADH2)
what are the 3 stages of respiration
Step 1: glycolysis and making acetyl Coa
Step 2: TCA cycle
Step 3: electron transport chain and oxidative phosphorylation
The electrons from glucose oxidation feed into the electron transport pathway, driving synthesis of ATP.
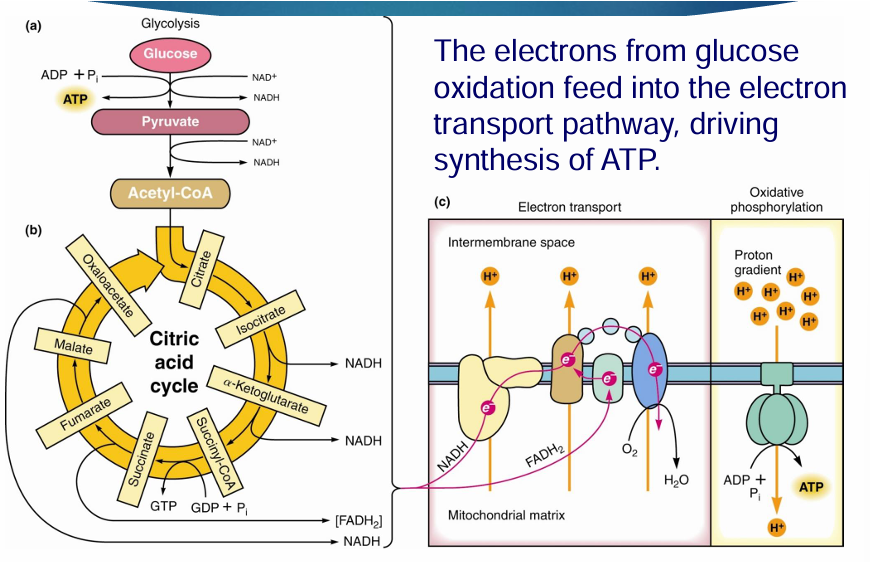
where does glycolysis occur (in cell)
cytoplasm of the cell
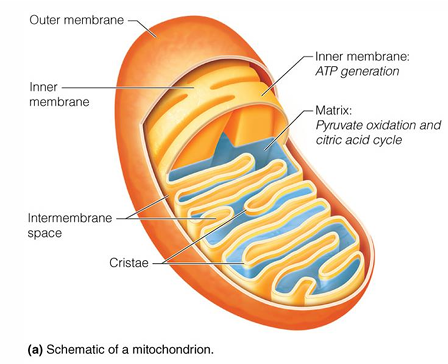
where does the citric acid cycle occur in cell
mitochondrial matrix
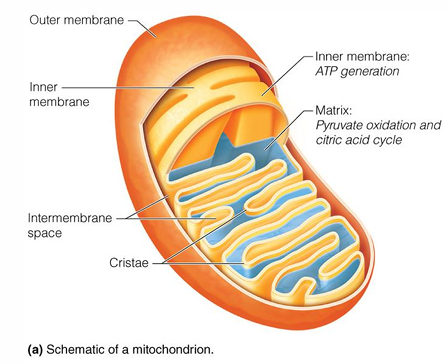
exception for part of TCA cycle that does not occur in mitochondrial matric
succinate dehydrogenase, which is located in the inner membrane
is part of complex 2 in the ETC
where does oxidative phosphorylation occur
inner membrane of mitochondria
tricarboxylate cycle (TCA) aka (2)
Krebs cycle and citric acid cycle
pyruvate from glycolysis is (what reaction and into what)
oxidatively decarboxylated to acetate
Acetate is acetyl-Coa without the Coa
acetate is then made into what
degraded into 2 CO2 in the TCA cycle
what is produced in the TCA cycle
2 CO2
1 GTP
3 NADH
1 FADH2
THIS IS PER ONE PYRUVATE
in TCA cycle, what is the product of the 8th reaction and also the reactant of the frist reaction
oxaloacetate (is recycled)
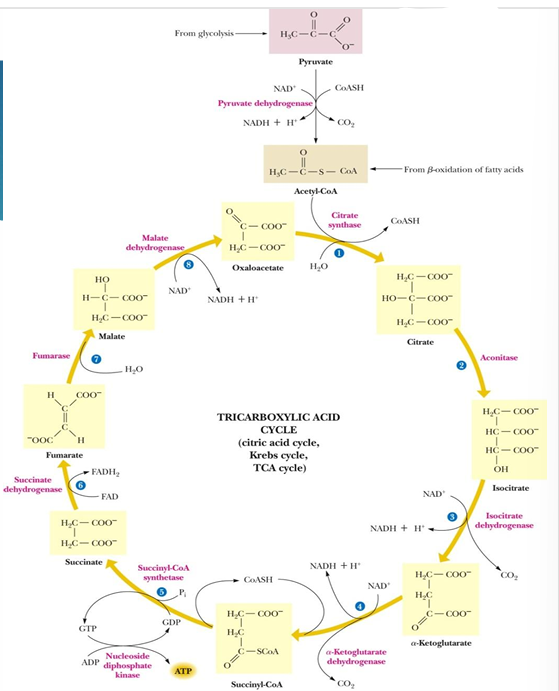
sequence of events in TCA cycle (9)
Prep: Convert Pyruvate to Actyl-CoA
Step 1: C-C bond formation to make citrate
Step 2: Isomerization via dehydration/rehydration
Steps 3–4: Oxidative decarboxylations to give 2 NADH
Step 5: Substrate-level phosphorylation to give GTP
Step 6: Dehydrogenation to give reduced FADH2
Step 7: Hydration
Step 8: Dehydrogenation to give NADH
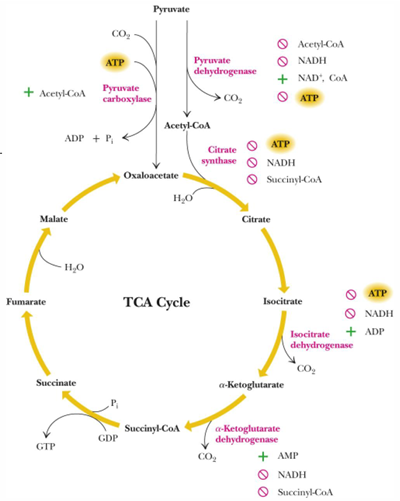
TCA cycle steps (substrate, products, and enzymes)
acetyl CoA + OAA—> citrate (citrate synthase)
citrate—> isocitrate (aconitase)
isocitrate —> alpha-ketoglutarate (isocitrate dehydrogenase)
alpha-ketoglutarate —> succinyl Coa (alpha ketoglutarate dehydrogenase)
succinyl Coa —> succinate (succinyl-Coa synthetase)
Succinate —> fumarate (succinate dehydrogenase)
Fumarate —> malate (fumarase)
malate —> oxaloacetate (malate dehydrogenase)
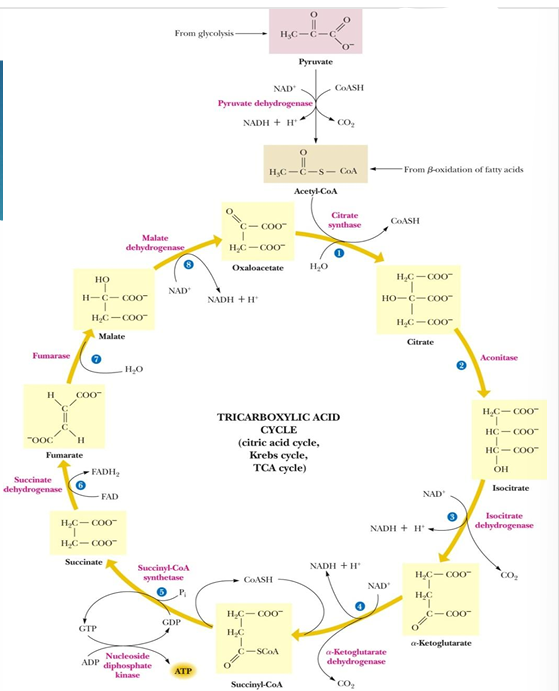
in which reactions is NADH made?
isocitrate —> alpha ketoglutarate by isocitrate dehydrogenase (3)
alpha ketoglutarate—> succinyl Coa by alpha ketoglutarate dehydrogenase (4)
malate—> oxaloacetate by malate dehydrogenase (8)
which reactions make FADH2
succinate —> fumarate by succinate dehydrogenase (6)
which reaction makes GTP
succinyl CoA —> succinate by succinyl CoA synthetase
what must pyruvate do to acess the TCA cycle
enter the mitochondria
how does pyruvate enter the mitochondria
by being converted into Acetyl-Coa
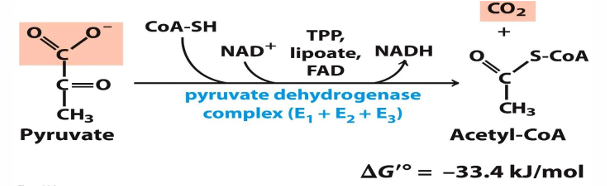
pyruvate —> acetyl CoA how (enzymes)
pyruvate dehydrogenase complex (PDC)
3 enzymes and 5 coenzymes
what occurs to pyrvate (what changes does it occur)
oxidative decarboxylase (so it has CO2 removed and a CoA added)
what are the fates for acetyl CoA (what can it do)
enter TCA cycle and produce energy
can be used to synthesize storage molecules
the pyruvate dehydrogenase complex (PDC) is what
a large multienzyme complex
the PDC consist of what
3 enzymes and 5 cofactors
what are the cofactors for the pyruvate dehydrogenase complex
Thiamin Pyrophosphate (TPP) – thiamin
Flavin adenine dinucleotide (FAD) – riboflavin
Coenzyme A (CoA) – pantothenic acid
Nicotinamide adenine dinucleotide (NAD) – niacin
Lipoate (aka lipoic acid)
thiamin pyruphosphate (TPP) comes from
thiamin
flavin adenosine dinucleotide (FAD) comes from
riboflavin
coenzyme A (CoA) comes from
pantothenic acid
Nicotinamide adenine dinucleotide (NAD) comes from
Niacin
what are the advantages of a multienzyme complex (4)
Short distance between catalytic sites allows channeling of substrates from one catalytic site to another
Substrate channeling minimizes side reactions
Regulation of activity of one subunit affects the entire complex
Activity of the complex is subject to regulation (ATP)
Pyruvate dehydrogenase complex is the prototype for (2)
α-ketoglutarate dehydrogenase
Branched chain α-keto acid dehydrogenase
These are practically the same in coenzymes and technique but differ in active sites as deal with different molecules
In reaction 1 of TCA cycle what happens (substrate—> product, enzyme)
oxaloacetate + Acetyl Coa —> citrate by citrate synthase
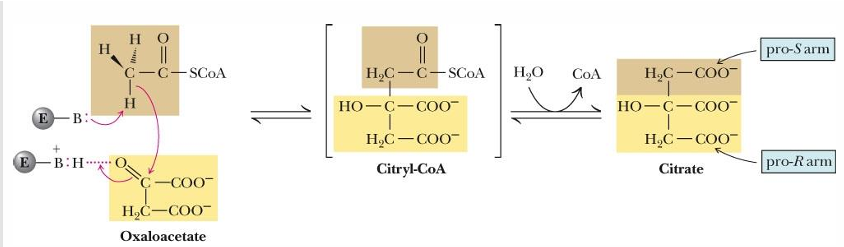
In reaction 1 of TCA cycle what kind of reaction occurs
condensation reaction
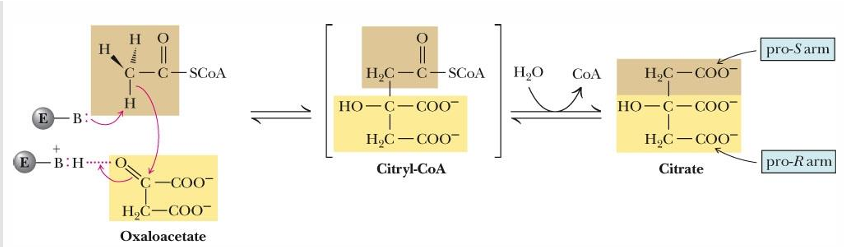
Reaction 1 of TCA cycle creates what (only place this occurs)
reaction with C-C bond formation
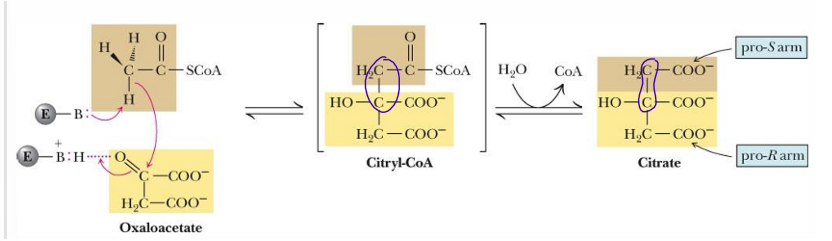
Reaction 1 of TCA cycle is considered the
limiting step of CAC, activity depends on [OAA]
because its activity is tightly regulated by the availability of substrates, the cell’s energy status, and feedback inhibition from its products
once reaction occurs, the cycle must occur
![<p><strong> limiting step</strong> of CAC, activity<strong> depends on [OAA]</strong></p><ul><li><p><em>because its activity is tightly regulated by the availability of substrates, the cell’s energy status, and feedback inhibition from its products</em></p></li><li><p><em>once reaction occurs, the cycle must occur </em></p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/d601421e-f984-4161-88ba-9c2724f197ff.png)
how is reaction 1 of TCA cycle inhibited
NADH and succinyl CoA are allosteric inhibitor
f we have high NADH, then that means we have lots oh high energy electron carriers that will provides lots of ATP. So if we have a lot we don’t need more
The accumulation of succinyl-CoA indicates that the cycle is operating and intermediates are being processed.
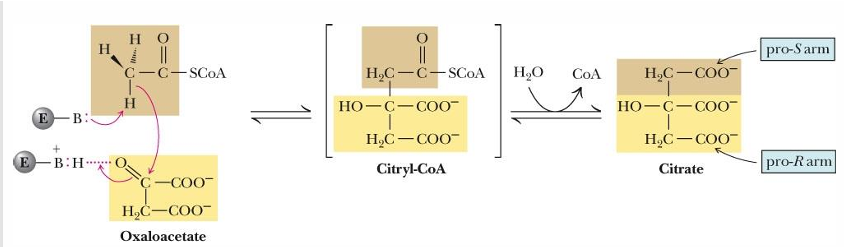
is reaction 1 of TCA reversible?
no!!!
which is why it is rate limiting, as once this reaction occurs it is committed to the cycle
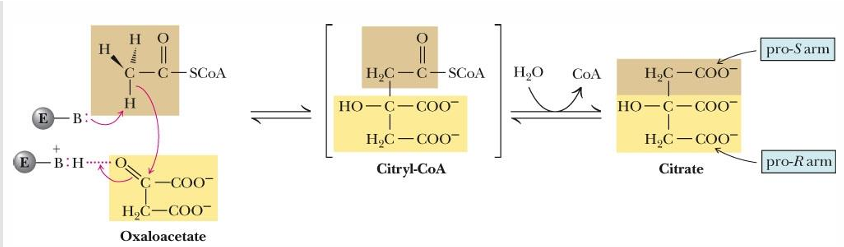
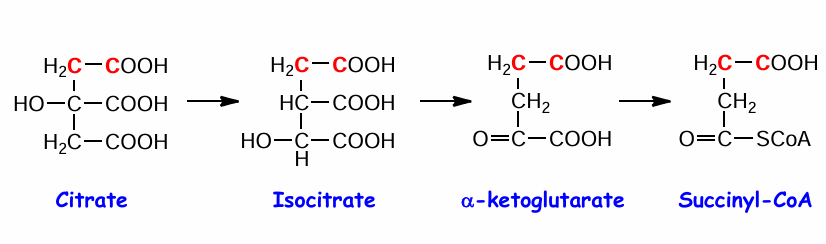
reaction 2 of TCA cycle (substrate, product, enzyme)
citrate —> isocitrate by aconitase
what occurs at reaction 2 of TCA cycle
(A) Elimination of H2O from citrate gives a cis C=C bond
(B) Addition of H2O to cis-aconitate is stereospecific
so H2O is removed and then added back to switch the OH and H location to the opposite
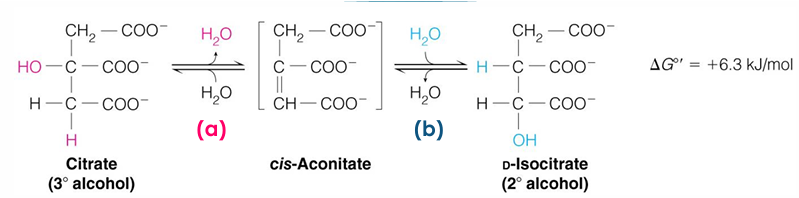
isocitrate is good substrate for what type of reaction
oxidation
will be able to take OH group from a alcohol to a ketone
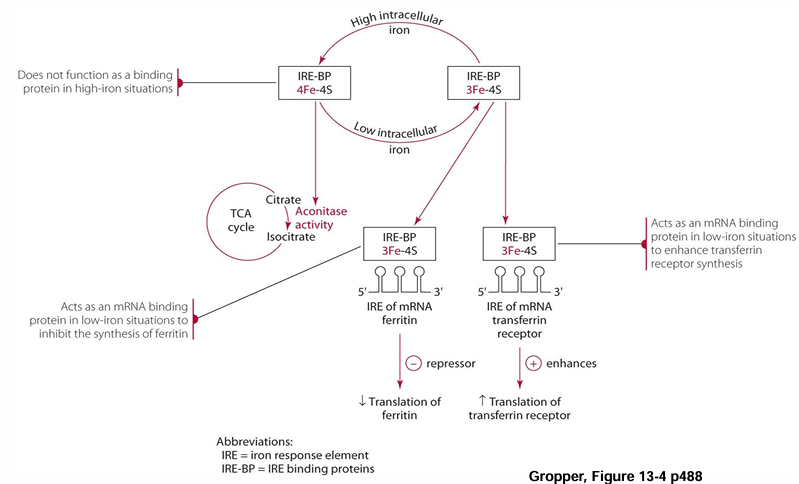
aconitase is involved is what (not to do with TCA)
iron status regulation!!!!
iron regulation
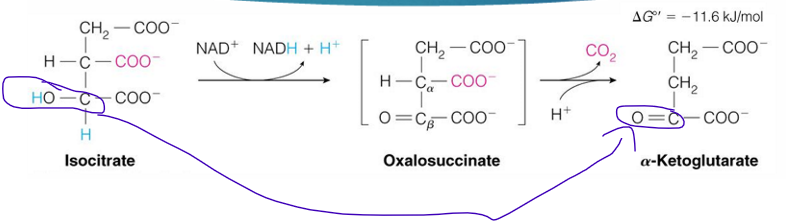
reaction 3 of TCA cycle (substrate, enzyme, product)
isocitrate —→ alpha ketoglutarate by isocitrate dehydrogenase
reaction 3 is what reaction (like what type of reaction)
oxidative decarboxylation
isocitrate dehydrogenase does what in reaction 3
oxidative decarboxylation so a CO2 is removed from isocitrate
the alcohol group is oxidized into a ketone which gives NAD+ an H to form NADH

the CO2 removed from isocitrate comes what?
the OAA part of it
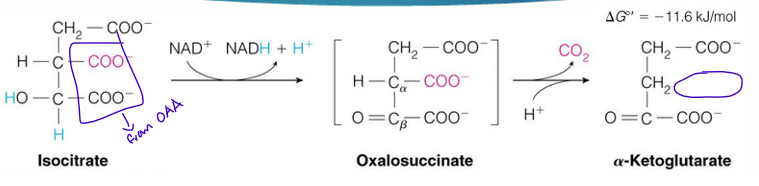
reaction 3 of TCA cycle makes what? (not substrate)
NADH
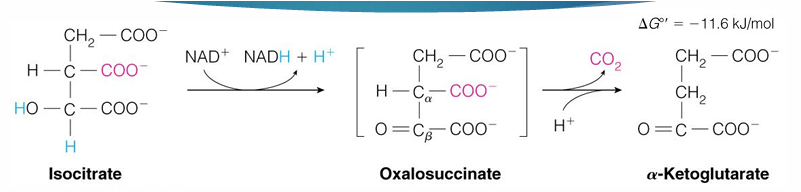
reaction 3 of TCA is reversible?
no
Irreversible because it is very exergonic so would require A LOT of energy to reverse with is not favorable
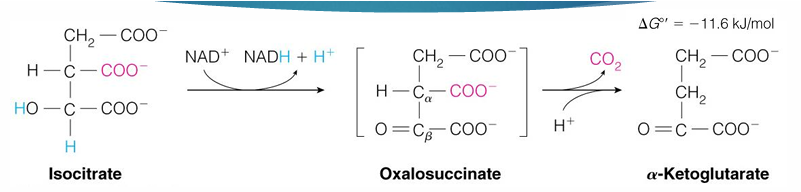
what is isocitrate dehydrogenase regulated by
product inhibition (alpha-ketoglutarate) and ATP
if lots of alpha-ketoglutarate, means we have a lot
if lots of ATP why make more
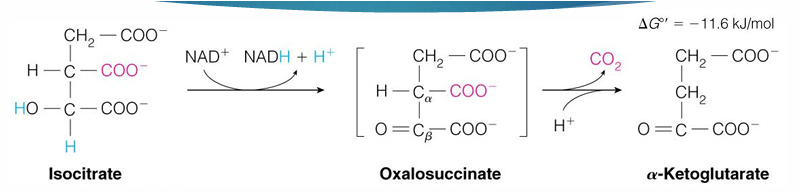
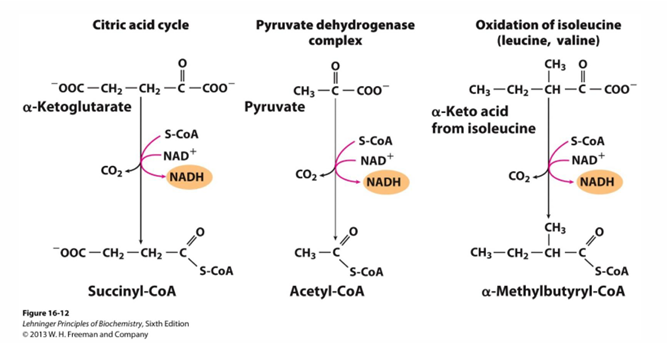
reaction 4 of TCA cycle (substrate, product, enzyme)
alpha ketoglutarate —> succinyl CoA by alpha ketoglutarate dehydrogenase
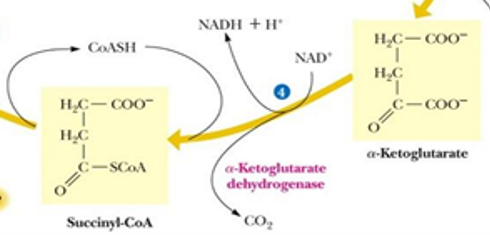
in reaction 4 by alpha-ketoglutarate dehydrogenase what has been made, released?
NADH is made
CO2 is released
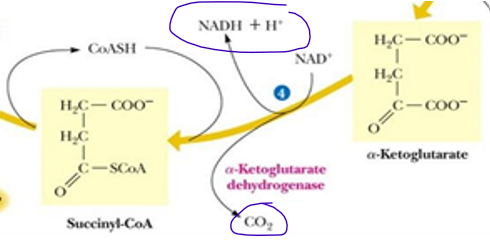
reaction 4 of TCA is the last
oxidative carboxylation reaction (release of last CO2)
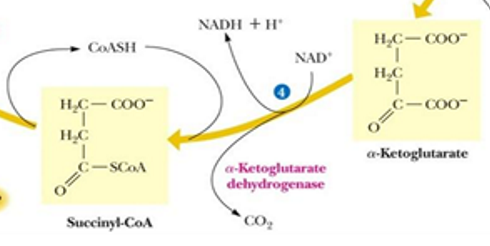
after reaction 4, it is considered that there is a what of glucose
net full oxidation (after 2 turns)
carbon lost at reaction 4 came from what original source
oxaloacetate
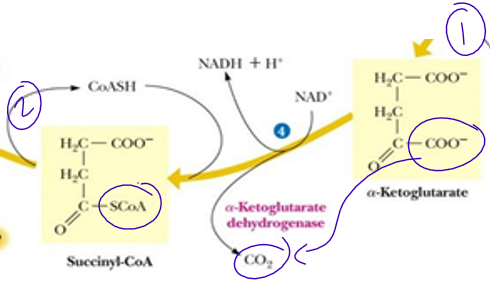
succinyl CoA is a (think energy)
high energy thioster bond (important for next reaction as will help form energy)
Hint of substrate level phosphorylation in the cycle
reaction 4 is reversible?
no, it is regulated by product inhibition
alpha ketoglutarate dehydrogenase is a complex similar to
pyruvate dehydrogenase (same coenzymes and identical mechanisms but differ in active sites to accommodate different sized substrates)
both the CO2 released from the citric acid cycle were derived from what molecule
oxaloacetate
reaction 5 (substrate, product, enzyme)
Succinyl CoA—> succinate by succinyl CoA synthase
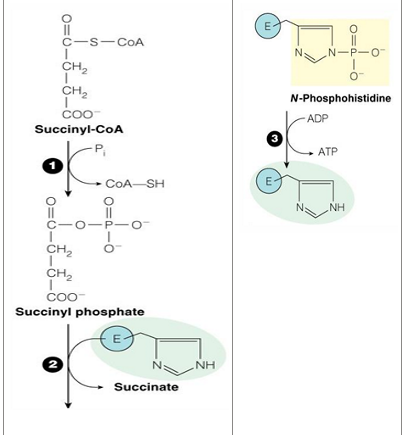
reaction 5 makes what
GTP (which can then be converted to ATP)
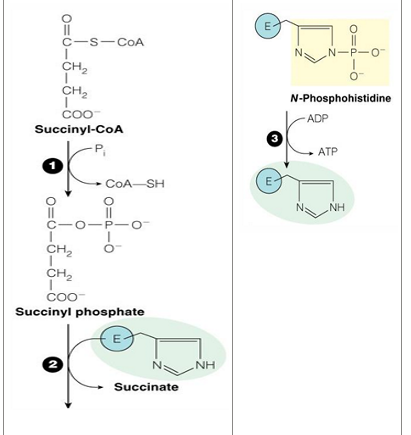
reaction 5 is what kind of reaction
substrate level phosphorylation
Energy of thioester allows for incorporation of inorganic phosphate
Goes through a phospho-enzyme intermediate
reaction 6 (substrate, product, enzyme)
succinate —> fumarate by succinate dehydrogenase
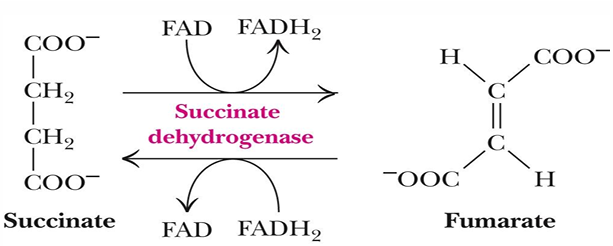
reaction 6 makes what (not product)
FADH2
succinate dehydrogenase is found where
Bound to mitochondrial inner membrane
Part of Complex II in the electron-transport chain!!!!!
reduction of succinate to fumerate requires
FADH2 (which then has its electrons passed to Coenzyme Q)
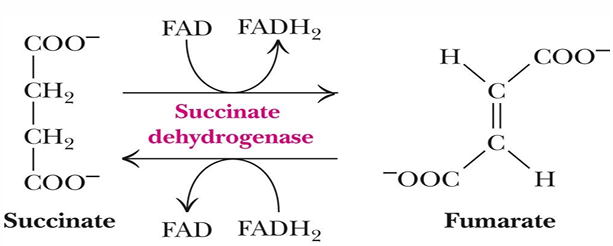
FAD relationship with succinate dehydrogenase
FAD is covalently bound to succinate dehydrogenase, unusual
So electrons immediately get fed into ETC at complex 2
reaction 6 (succinate dehydrogenase) is inhibited by
malonate (structural analog of succinate)
it is a competitive inhibitor that that will prevent the formation of fumarate and lead to buildup of succinate
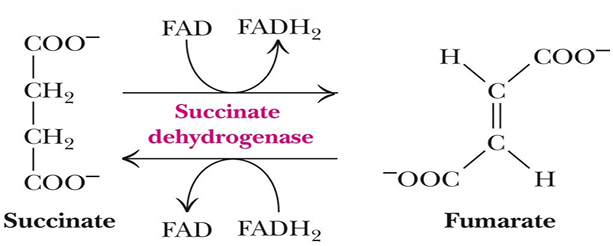
reaction 7 (substrate, enzyme, product)
fumarate —> malate by fumarase
what occurs at reaction 7
Stereospecific (so makes one specific stereoisomer though others could also be made)
Addition of water is always trans and forms L-malate
OH- adds to fumarate
Then H+ adds to the carbanion
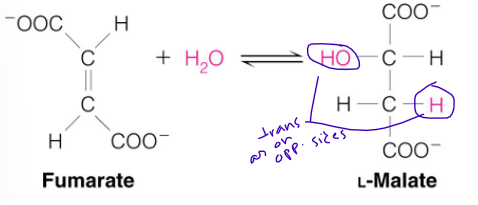
reaction 8 (substrate, enzyme, product)
malate —> oxaloacetate by malate dehydrogenase
in reaction 8, we regenerate what product
oxaloacetate for citrate synthase to use
reaction 8 creates what (not product)
NADH
oxaloacetate is kept at what levels in cell, why
kept VERY LOW by citrate synthase
Continuously used to make citrate
Pulls the reaction forward instead of going backwards to malate as it is reversible step!!!
the carbon atoms of acetyl CoA have different
fates
what are the 2 carbons we are referring to
carboxyl carbon and methyl carbon of acetyl CoA
carbonyl C of acetyl CoA becomes CO2 when
The carboxyl carbon atom of acetate is released as CO2 in the second turn of the cycle (following entry of acetyl CoA)
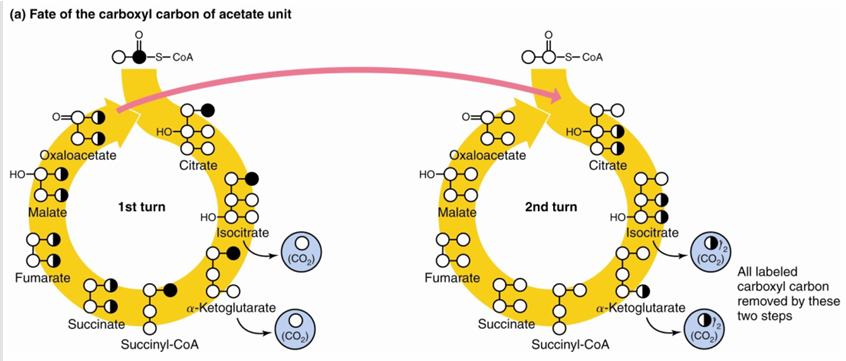
methyl carbon of acetyl CoA is removed as CO2 when
Release of the methyl-C of acetate as CO2 requires multiple turns of the cycle (4 turns)
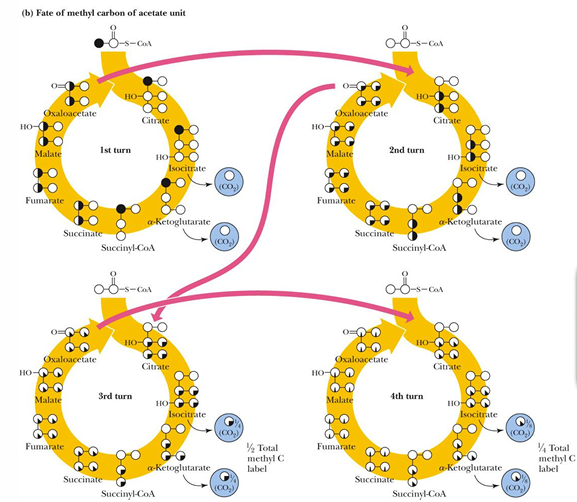
one turn of the TCA cycle makes what
3 NADH
1 FADH2
1 GTP —> ATP
releases 2 CO2
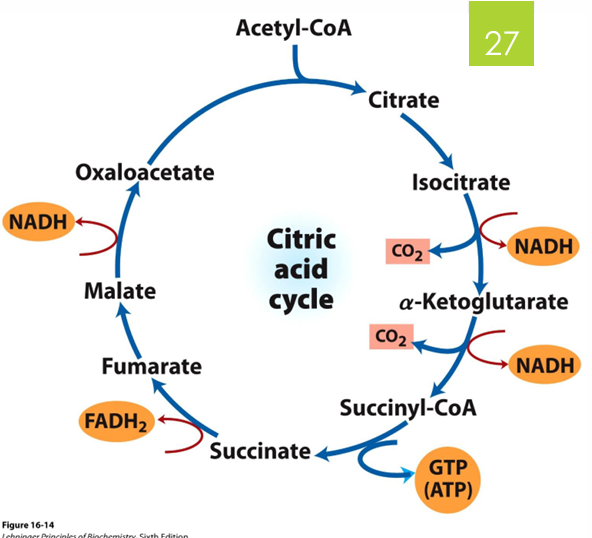
because glucose is broken down into 2 pyruvates, how many TCA cycles does a glucose molecule go through
2
what reactions of the TCA are irreversible
acetyl CoA + OAA—> citrate by citrate synthase (1)
isocitrate—> α ketoglutarate by isocitrate dehydrogenase (3)
α ketoglutarate —> sucinnyl CoA by α-ketoglutarate dehydrogenase (4)
one TCA cycle has net oxidation of
2 carbons to CO2
Equivalent to two carbons of acetyl-CoA
but NOT the exact same carbons as it is the OAA carbons that are released
In TCA cycle, energy is captured by
electron transfer to 3 NADH and 1 FADH2
in 1 TCA cycle we make what that is then converted to ATP
GTP (through substrate level phosphorylation)
in TCA cycle, intermediates are not
depleted but constant being regenerated so that reaction can keep going
direct and indirect ATP yield (how much ATP, NADH, and FADH from complete oxidation of 1 glucose are made)
10 NADH
2 FADH2
4 ATP
the TCA cycle provides intermediates for what
intermediates for biosynthesis
other pathways
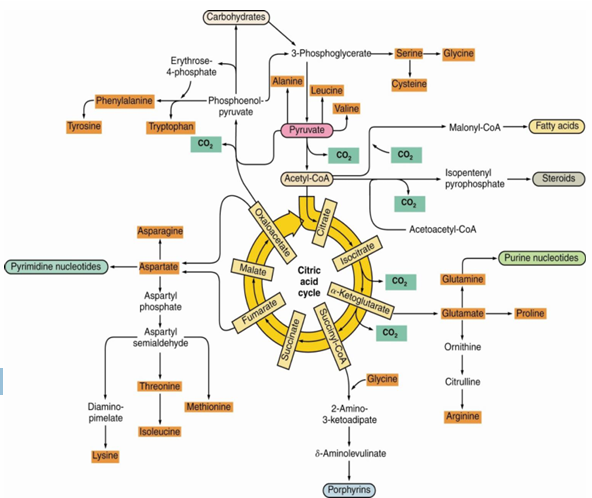
what are anapleurotic processes
(“filling up”) processes are required to restore these intermediates so the TCA can continue
-The TCA cycle (Citric Acid Cycle) is a central hub of cellular metabolism, and it involves a number of intermediates (such as citrate, α-ketoglutarate, succinate, malate, etc.) that are used in various biosynthetic processes. However, some of these intermediates may be drawn off the cycle for the synthesis of amino acids, nucleotides, or other biosynthetic molecules, potentially leading to a depletion of these intermediates. Anapleurotic reactions help refill the TCA cycle with intermediates to ensure that it can continue functioning effectively. Without these replenishing reactions, the cycle could become "blocked" or inefficient, limiting energy production.
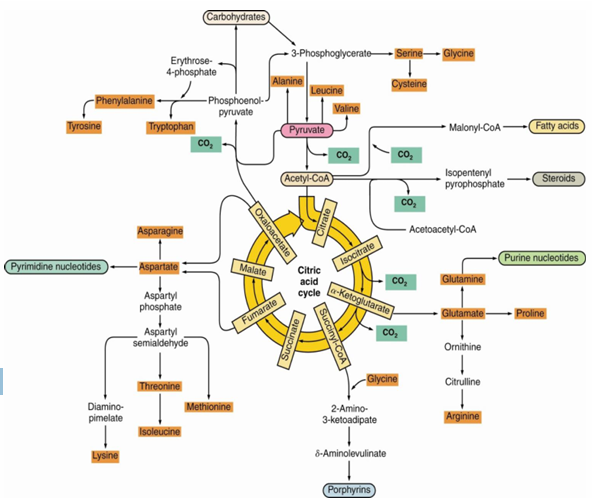
what is the most important antipleuritic reaction of the TCA cycle
OAA
Intermediates in the citric acid cycle can be used in
biosynthetic pathways (removed from cycle to be used for other anabolic pathways)
we must ____________ intermediates in order for cycle and central metabolism to continue
replenish
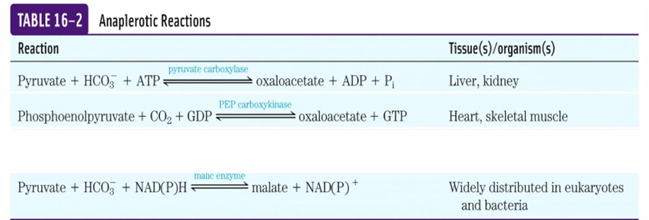
4-carbon intermediates are formed by carboxylation of
3-C precursors
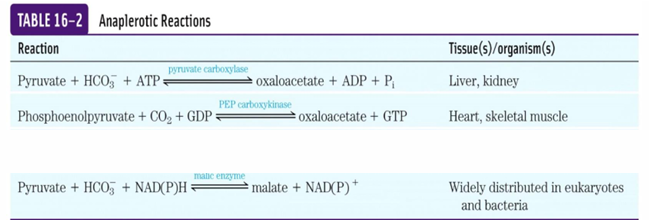
transamination reaction is what, does what
amino acid—> regenerate/ fill up CAC intermediate
Part of first step of amino acid metabolism
We remove amino group from amino acid to a keto acid, and then the keto acid becomes amino acid and other one is now a keto acid
pancreas releases insulin as response to
high glucose in the bloodstream
Recent research has shown that anaplerotic enzymes feed alternative pathways that (based on insulin)
produce cytosolic signal molecules that also support insulin secretion
Pancreatic β-cells maintain high levels of
the anaplerotic enzyme pyruvate carboxylase
what is The pyruvate/malate cycle (where it occurs, what is the cycle, what is made that is of importance to insulin)
-in pancreatic β cells.
-cycle where Oxaloacetate produced by high levels of pyruvate carboxylase is converted to malate, then exported to the cytosol
-then it can be made into pyruvate, enter mitochondria and become OAA again
-The NADPH produced by turning Malate into pyruvate act as intracellular messengers that appear to be as significant as ATP in provoking insulin release
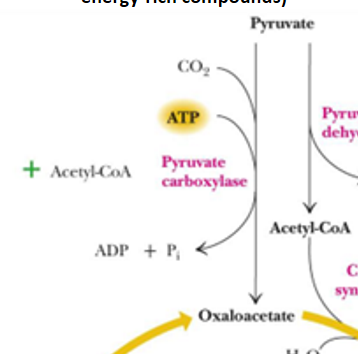
pyruvate carboxylase does what and how does it relate to insulin
In these cells, half the pyruvate from glycolysis is converted by pyruvate carboxylase to oxaloacetate, much of which is converted to malate and exported to the cytosol, as part of a pyruvate/malate cycle
NADPH and other metabolites generated from the pyruvate/malate cycle act as intracellular messengers that appear to be as significant as ATP in provoking insulin release
what is pyruvate carboxylase
enzyme that turns pyruvate into oxaloacetate
regulation of TCA cycle occur by controlling what (what do we target- 2 things)
Entry into the cycle (PDH-pyruvate dehydrogenase- and citrate synthase)
key irreversible reactions (isocitrate dehydrogenase and α ketoglutarate dehydrogenase)
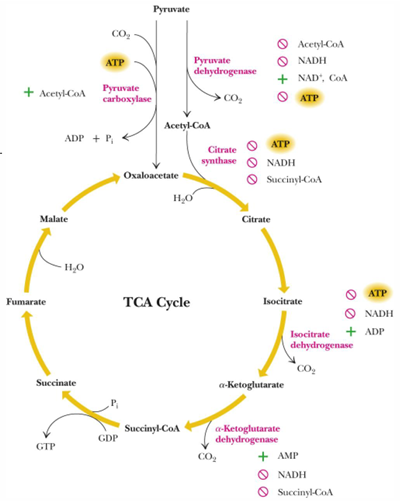
cycle lux is primarily controlled by what 3 things
Allosteric activation of isocitrate dehydrogenase by ADP (energy state)
NAD+/NADH ratio (redox state)
Inhibition of relevant enzymes by acetyl-CoA and succinyl-CoA (availability of energy-rich compounds)