Preparing soluble salts acid and alkali
1/5
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
Apparatus
Burette, retort stand and clamp
Pipette (25cm3) and safety pipette filler
Conical flask
Phenolphthalein indicator
Hydrochloric acid
Sodium hydroxide solution
Decolourising charcoal
Evaporating basin
Filter funnel and filter paper
Bunsen, tripod and gauze
Method
Fill burette with hydrochloric acid
With pipette filler, place 25cm3 sodium hydroxide into conical flask
Add 3 drops phenolphthalein indicator to conical flask
Add hydrochloric acid from burette, swirling gently until colour changes
Record volume of hydrochloric acid added
Repeat without indicator, adding recorded volume or use charcoal to remove indicator
Pour solution from conical flask into evaporating basin
Heat filtrate on tripod and gauze until volume is half of original
Leave basin aside to allow solution to cool and crystalise
Filter off crystals (if necessary)
Dry between two sheets of filter paper or desiccator/ low temp oven
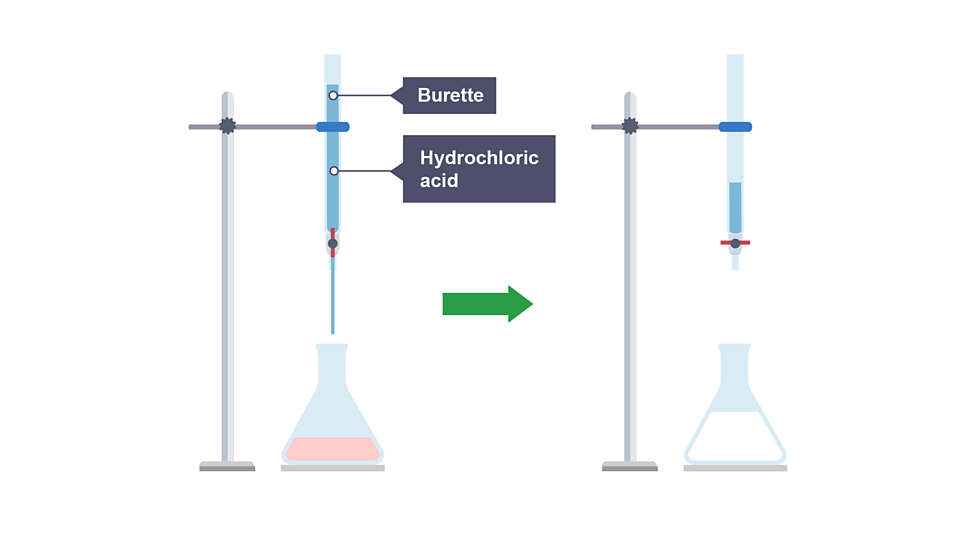
Observations
colourless solutions
indicator changes from pink to colourless when neutralised
colourless filtrate when using charcoal
white crystals
Equation
hydrochloric acid + sodium hydroxide → sodium chloride + water
Error
incomplete neutralisation, solution wont be pure
Safety
safety goggles to protect eyes from splashes
handle in well ventilated area/ fume cupboard
avoid skin contact with corrosive sulfuric acid using gloves