DDS Lecture 14 Content
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
General Manufacturing Process
Procurement and selection of components and containers
Product preparation: in sterile environment (aseptic)
Quality assurance (stability testing, pyrogen testing)
packaging and labeling
Components of Parenterals
vehicles
solutes
Parenteral Vehicle
non-irritating
non-toxic
no pharmacologic activity
no effect on active ingredient
physical properties: stability at various pH, temperatures, viscosity, miscibility with body fluid
Aqueous Vehicles
isotonic
drugs may be added
0.9% Sodium Chloride injection
Dextrose
Lactated Ringers
Water-Miscible Vehicles
used to affect solubility and reduce hydrolysis
propylene glycol (phenytoin)
Non-aqueous vehicles
fixed oils of vegetable origins
ex. soybean oil (used for propofol), amphotericin B liposomal
Parenteral solutes
antimicrobial agents: thimerosal 0.01%, benzethonium chloride 0.01%
buffers: citrates, acetates, phosphates
antioxidants: sodium bisulfite 0.1%
other added substances: sodium benzoate
Parenteral containers
no container is totally insoluble
should avoid
leaching
permeation
adsorption
selection of containers:
glass
plastic polymers: polyethylene, polypropylene, polyvinyl chloride (PVC)
rubber polymers- butyl, silicone, natural rubber
Plastic containers
Do not break
weight reduction (very light)
flexible (dropper)
risk of permeation, leaching, adsorption: must carefully select drugs
not clear: difficult to inspect contents
melts under thermal sterilization
Glass container
glass composition: silicon dioxide and other oxides
oxides loosely bound to silicon oxide tetrahedron
4 main types, 3 used in containers
Type I Glass
silicon dioxide and boric acid
neutral glass
preferred
low levels of migratory oxides, low leaching
suitable for all products
more expensive
Type II Glass
sodium oxide and calcium oxide
soda-lime glass with high hydrolytic resistance
melt at lower temperatures
lower concentration of migratory oxides than type III
treated with sulfur dioxide to dealkalize interior surface of glass
best used for solutions with pH < 7 or is buffered
Type III glass
sodium oxide and calcium oxide
soda-lime glass with moderate hydrolytic resistance
highest concentration of migratory oxides
best used for dry substances, non-aqeous preparations
Industrial Preparation of Parenteral Products
Compounding
Filtration
Filling/Sealing
Sterilizing
Methods of Sterilization
steam
dry heat
filtration
gas
ionizing radiation
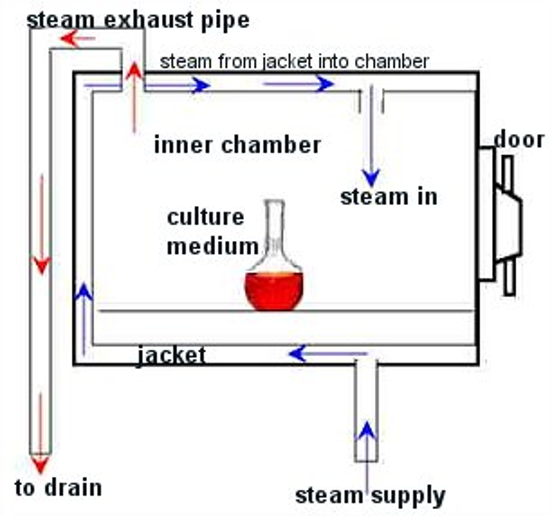
Steam Sterilization
equipment used: autoclave
with moisture, bacteria coagulate and are destroyed at lower temperature (vs. without moisture)
bacterial cells with large % of water killed more easily (spores difficult)
mech of action: denaturing and coagulation of bacteria’s essential protein
ideal for preparations that can withstand required temperatures, but not affected by moisture
usually method of choice is feasible
widely used settings: 15 lbs pressure, 121.5 C (250 F), 20 minutes
used for solutions sealed in ampules or vials, surgical instruments, bulk solutions
Dry Heat
electric or gas “ovens”
dry heat is less effective in killing than moist heat
mechanism of destruction is dehydration
conducted at 150-170 C (302-338 F) for 2-4 hours
higher temp, longer than steam
effective for substances not effectively sterilized by moist heat
ex.
fixed oils
glycerin
mineral oil
paraffin and zinc oxide
thermostable powders
glassware, surgical instruments
Filtration
removal of microbes by adsorption via filter medium
filters produced according to pore size
millpore 14 - 0.025 mcm, smallest bacteria is 0.2 mcm
electric charge of microbes, pH of solution, temperature
best used for heat sensitive solutions (thermolabile), low viscosity solutions
Types of Filters
Candle
Asbestos
Sintered Glass
Membrane (MOST COMMON)
Membrane filter
Bubble Point Test
tests filter integrity
minimum pressure required to force liquid out of the capillary space in the membrane
smaller the pore size, the high the bubble point pressure
manufacturer gives standards for filter
will give certain threshold for pressure
should not get pressure lower than standard (means filter is broken)
Gas Sterilization
use of ethylene oxide or propylene oxide gas
thought to inhibit bacterial cell wall formation
extremely flammable when mixed with air
sterilization with ethylene oxide
~4-16 hours
con: greater precautions required because variability of time, temperature, gas concentration, and humidity
also flammability
pros:
great penetrating qualities useful for powders and heat labile enzyme preparations
antibacterials
ophthalmic prep
vaginal inserts
plastic syringes and tubes
Ionizing Radiation
sterilization via gamma (Cobalt-60), cathode rays, UV lamp
application limited due to highly specialized equipment and effects of radiation
thought to destroy vital chemicals and/or structures (ex. chromosomal nucleoproteins)
used for antibiotics, hormones, sutures, prepackaged, disposable items
Pyrogens
lipopolysaccharide metabolic products from outer cell walls of gram-negative organisms
water soluble and thermostable
may remain after steam and filtration sterilization
bacterial endotoxin unit limit (USP)
5 EU/kg/hour for most drugs
Intrathecals: 0.2 EU/kg
EU = endotoxin units
Pyrogen Testins
Rabbit Test
LAL test
Rabbit Testing
Instruments heated to 250 C > 30 min
heat product to be tested to 37 C ± 2
inject product into vein of 3 rabbit’s ears 10 ml/kg and record temperature for 3 hours
If no rabbit shows increase in temperature >0.5 C OR sum of 3 rabbits is less than 1.4 C, meets USP standards
If one rabbit shows increase in temp more than 0.5 C, repeat test on 5 more rabbits
If not more than 3/8 show rise in temperature or 0.5 C, or sum of 8 rabbits is less than 3.3 C, meets USP standards
LAL test
Limulus Amebocye Lysate Test
extract blood cells of horseshoe crab (Limulus polyphemus) contains enzyme and proteins that coagulate in presence of low levels of lipopolysaccharides
aka Gel clot test, photometric test
Used by USP
5-50x more sensitive to endotoxin than rabbit test
some drugs interfere with test: meperidine, promethazine, oxacillin, vancomycin
Sterility Testing
USP 71: Must confirm sterility of each sterilized batch by either direct innoculation or filtration
use culture medium trypticase soy broth (TSB) and fluid thioglycollate medium (FTM)
medium incubated for 14 days
does require validation before product shipped
direct innoculation or membrane filtration test
Direct inoculation sterility test
the product/medical device will be in direct contact with the test media throughout the incubation period
Membrane Filtration Sterility Test
Test sample effluent is transferred to a cellulose nitrate or cellulose acetate membrane filter capable of retaining microbe
filter is then transferred to two specific nutrient test mediums and incubation for 14 days
Biological Indiactor
biological marker
provides information on whether necessary conditions were met to kill a specific number of microbes for a given sterilization process, providing a level of confidence in the process
endospores or bacterial spores primarily used in biological indicator
microbes considered some of toughest to kill
Steam biological marker
Bacillus stearothermophilus
Gas biological indicator
Bacillus stearothermophilus
Dry Heat Biological Marker
Bacillus subtilis
Ionizing Radiation Biological Marker
bacillus pumulis
bacillus stearothermophilus
bacillus subtilis
USP 797
USP has 2000 chapters
797: minimum practice and quality standards to which sterile preparations should be compounded
applicable for all settings and all personnel that compound sterile preparations
ANY healthcare setting, not just pharmacy
enforced by state boards of pharmacy and FDA
was made official in June 2008
Sterile Preparations
injections (IV, TPN, IM, SC, epidural, IT)
ophthalmic drops and ointments (ex.
aqueous pulmonary inhalations
baths and soaks
irrigations for internal body cavities
implants
Class 5 Environment
class 100
no more than 100 particles per cubic foot
inside hood
Class 7 Environment
class 10,000
no more than 10,000 particles per cubic foot
clean/buffer room
Class 8 Environment
Categories of Compounding sterile preparations
4 main categories:
Immediate use
category 1
category 2
category 3
classifications based on potential for microbial, physical, or chemical contamination during compounding
determined by where the compounded sterile preparation is made and the time period within which it must be used
if the compounding personnel are unsure, they should go with the more stringent category to ensure safety
Immediate Use CSP
ISO Class 5 Environment not required
7 requirements:
Aseptic standard operating procedures and process must be in place
Personnel trained and demonstrate facility-specific competency in aseptic technique
preparation performed per FDA-approved labeling or evidence-based information
preparation limited to 3 different sterile products
any unused medication from a single dose vial is discarded
administration begins within 4 hours of preparation
BUD: 4 hours
CSP must be labeled unless it is administered by, or the administration is witnessed by, the preparer
ex. prep of epinephrine drip at bedside by nurse of pharmacist for patient in cardiac arrest
Category 1 CSP
must be compounded in an ISO Class 5 air quality environment or better
Class 5/PEC does not have to be located within Class 7 buffer room
can be in segregated compounding area (SCA)
ex. PEC located directly in regular pharmacy satellite
PEC: primary engineering control
max BUD of 12 hours at room temp, 24 hours in fridge
short bc of higher risk for contamination when there’s no buffer or ante room
Category 2 CSP
preparation compounded in PEC, within buffer room, with ante-room separating the buffer room from the rest of the pharmacy
assigned BUD based on whether or not they are produced from sterile products only or produced from non-sterile products but terminally sterilized (ex. sterilization after the CSP has been produced using dry heat, steam, irradiation), and whether or not sterility testing has been performed on them
Category 3 CSP
allows for longest dating
requirements in addition to those for Cat2 to ensure safety
additional requirements: increased frequency of aseptic manipulation competency, sterile garbing, increased viable air and surface sampling, and increased frequency of sporicidal applications during cleaning
BUDs must be supported by stability data obtained using a stability-indicating analytical method
Sterility testing and endotoxin testing required for all cat3
Multi-dose Vial
contains preservatives
max BUD: 28 days after entering with needle or puncturing stopper
if any type of contamination of multi-dose vial is suspected, discard immediately
Single-dose vial
max BUD: 12 hours after first entry
if vial is entered in at least ISO class 5 environment
may be removed from ISO class 5 PEC for storage at appropriate temp during 12 hour period
once ampules have been opened, they must not be stored for any period of time, regardless of air quality
Sources of ISO-Class 5
Laminar airflow
Horizontal
Vertical
good when drug is hazardous, do not have molecules to flow toward you
continuous flow of air through HEPA filter (air filter that removes 99.97% of particles with size of 0.3 microns)
compounding aseptic isolator
Hazardous drug preparation
present health risk to compounding personnel
should be prepared in an ISO class 5 environment in an ISO class 7 negative pressure area physically separated from other prescription areas
negative pressure: so air from inside room doesn’t escape; air from outside will come in when person enters
Proper Hygiene and Garbing
Prior to entering buffer area or segregated compounding area