intestinal protozoa quiz - microbio II (cls 54
1/79
Earn XP
Description and Tags
amoebas, flagellates, cilliates, coccidia, microsporidia galore!
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
80 Terms
amoeba general characteristics
Unicellular
Eukaryotic (more complex than bacteria)
transmission: ingestion of cysts from food and water contaminated with fecal material
reproduce by BINARY FISSION (asexual)
after replicating its genetic material through mitotic division
cell divides into two equal-sized daughter cells
list of amoebas (8)
Entamoeba histolytica
Entamoeba dispar
Entamoeba hartmanni
Entamoeba coli
Entamoeba polecki
Entamoeba nana
Iodamoeba bütschlii/butschlii/buetschlii
Blastocystis sp (hominis) (not really an amoeba technically)
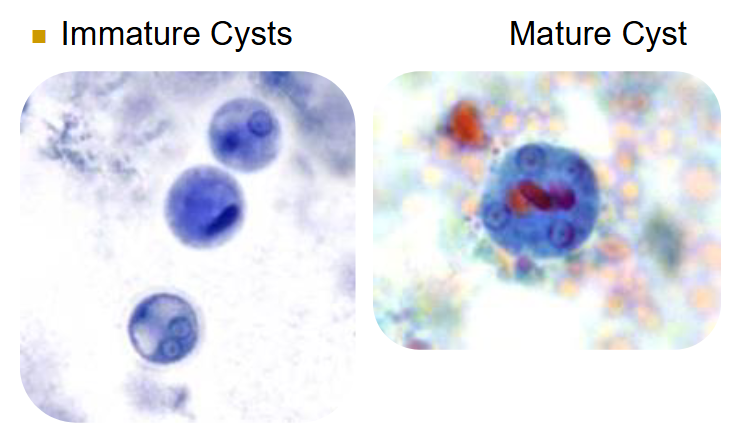
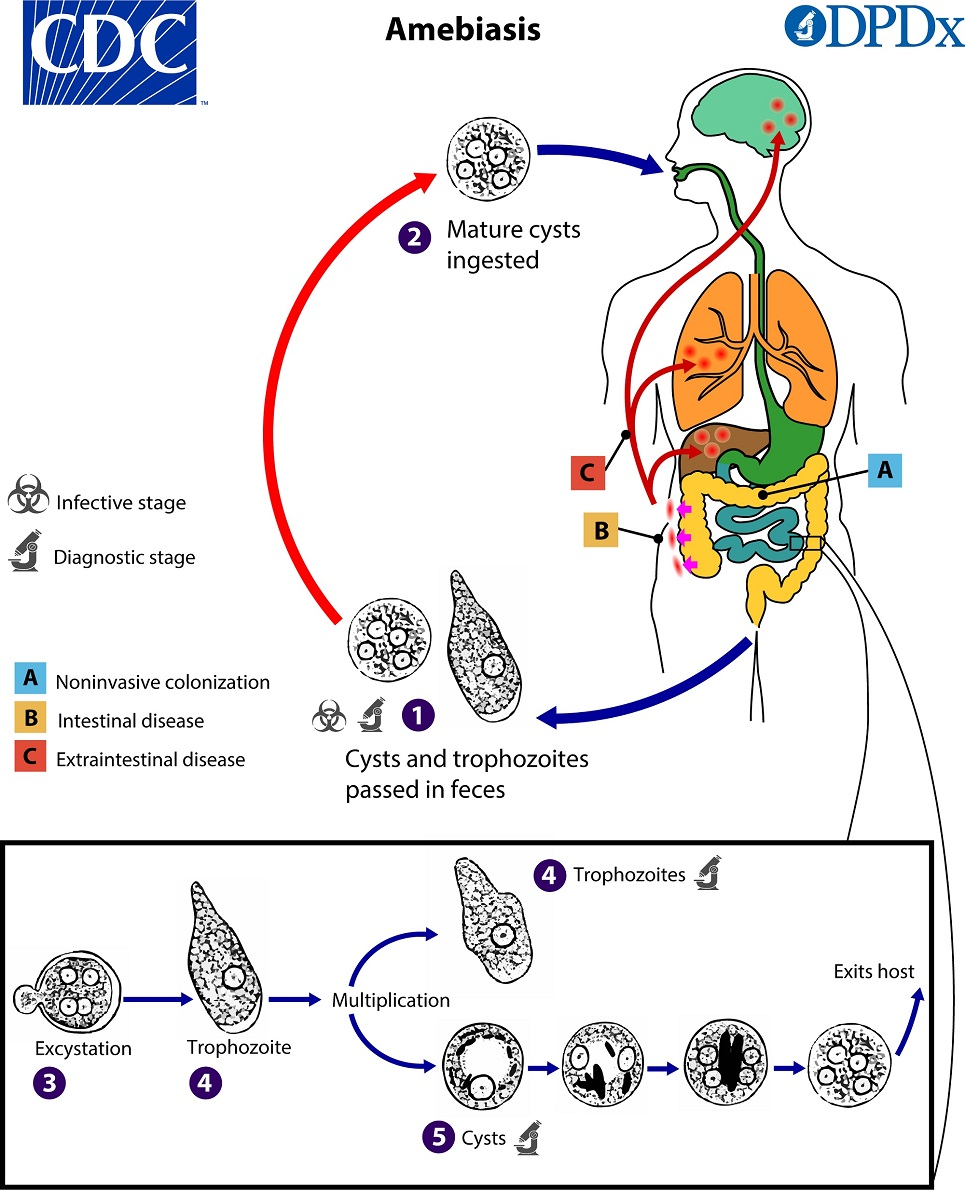
amoebic life cycles (general)
cysts passed in feces
mature cysts ingested (infective & diagnostic stage)
in the body:
excystation → trophozoite
trophozoite multiplicates into another troph and/or cyst
cyst undergoes multiple stages before exiting body as a mature cyst
(amoeba) entamoeba histolytica
one of world's most important parasites due to its worldwide distribution
only pathogenic intestinal amoeba
Infants rarely harbor this parasite
Causes multiple types of infection
Gastroenteritis/non-dysenteric amoebiasis
Amoebic dysentery
Amoebic abscess
Amoeboma
Sometimes confused with intestinal carcinoma
(entamoeba histolytica) amoeboma
a rare form of invasive amoebiasis
Associated with abscesses
Granulomatous mass with a fibrous periphery and inflammatory center
May cause bowel obstruction and intermittent bleeding
Easily confused with colon cancer; must be histologically differentiated
(entamoeba histolytica) non-dysenteric infection
Asymptomatic to mild
Often a chronic infection
Symptoms: abdominal pain, nausea, flatulence, irregularity, headaches, fatigue, nervousness
(entamoeba histolytica) amoebic dysentery
Amoebas eat into intestinal tissues
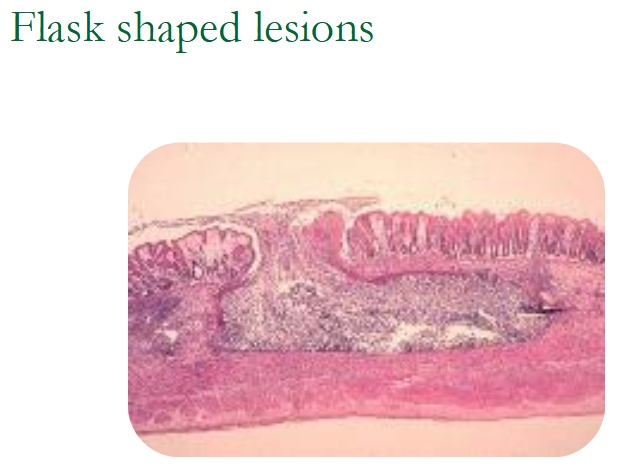
Form flask shaped ulcers
Can perforate the intestine
Stools consist of blood and mucus--eats RBCs
"pot bound"
No fever
(entamoeba histolytica) amoebic abscesses
Organism carried via portal circulation to liver
Form sterile abscesses
No living orgs inside abscesses
Amoebas are in the abscess margins
Lungs are a secondary site
Patients lack intestinal symptoms
10% of untreated cases developed abscesses
(entamoeba histolytica) pathogenicity
Coinfections by bacteria that cause GI disease may occur
Pathogenicity related to strain of amoeba
Possible hyaluronidase production contributes to pathogenicity
Low protein diets enhance severity
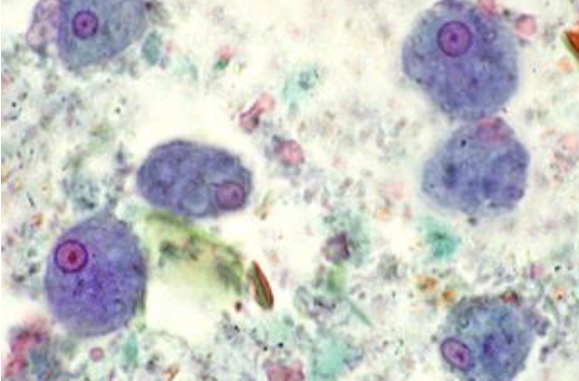
entamoeba histolytica trophozoite
Thin, delicate chromatin ring
Central, compact karyosome
Ingested RBC
Not always present
Size 20 u (usually 15-20; can be 10-60u)
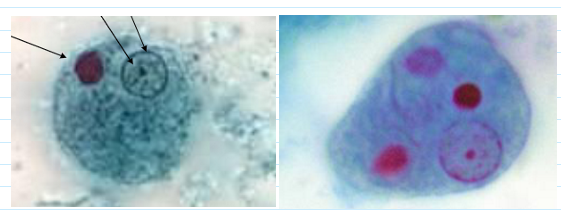
entamoeba histolytica cyst
Up to 4 nuclei in a cyst—looks the same as the troph nucleus
Smooth ended chromatid bar
Easier seen on trichrome
10-15um (can be up to 20u)
entamoeba histolytica life cycle
cysts and trophozoites are passed in feces (cysts in formed stool; trophs in diarrhea)
excystation occurs in the small intestine and trophozoites are released, which migrate to the large intestine
trophozoites may remain confined to the intestinal lumen or invade the intestinal mucosa, or blood vessels, reaching extraintestinal sites such as the liver, brain, and lungs
trophozoites multiply by binary fission and produce cysts and both stages are passed in the feces
(amoeba) entamoeba dispar
Morphologically identical to E histolytica
Does NOT ingest RBCs
Commensal
Need serological tests to differentiate
Rapid EIA tests for E histolytica
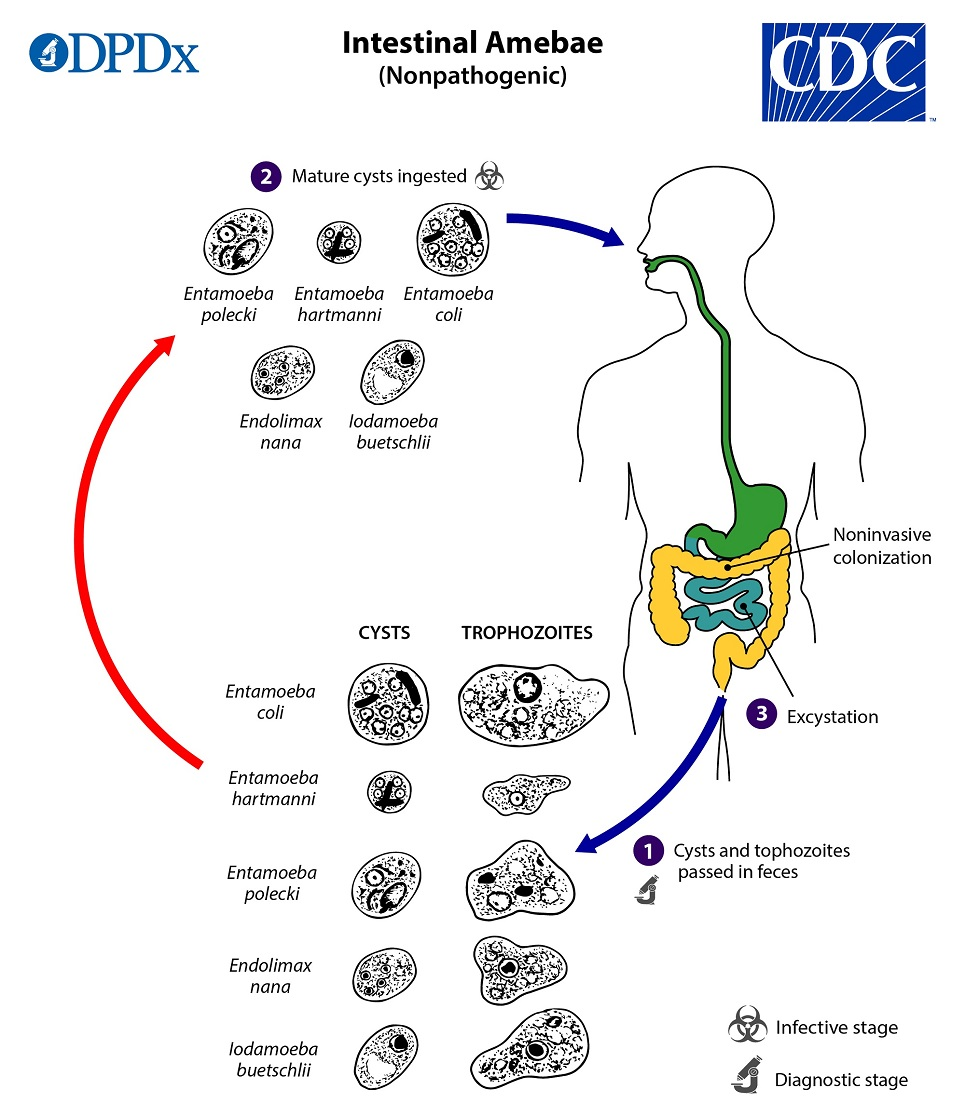
life cycles for Entamoeba coli, E. hartmanni, E. polecki, Endolimax nana, and Iodamoeba buetschlii
all considered nonpathogenic
both cysts and trophs of these species are passed in stool and considered diagnostic
cysts found in formed stool, whereas trophozoites found in diarrhea
excystation occurs in the small intestine and trophozoites are released, which migrate to the large intestine
trophozoites multiply by binary fission and produce cysts, and both stages are passed in the feces
because of the protection conferred by their cell walls, the cysts can survive days to weeks in the external environment and are responsible for transmission
entamoeba harmanni trophozoite
Looks the same as E histolytica except for size
8-10u (5-12u)
extremely tiny
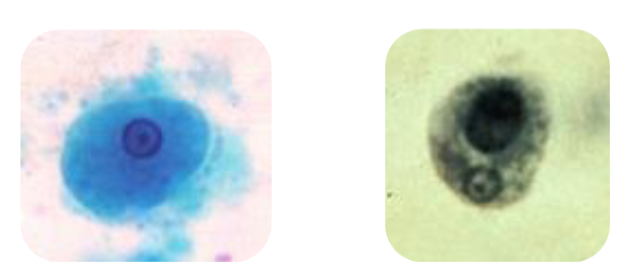
entamoeba hartmanni cysts
Same as E histolytica except for size
6-8u (5-10u)
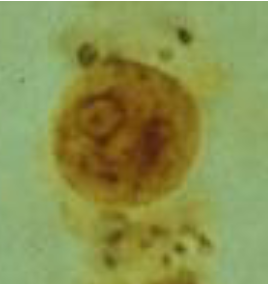
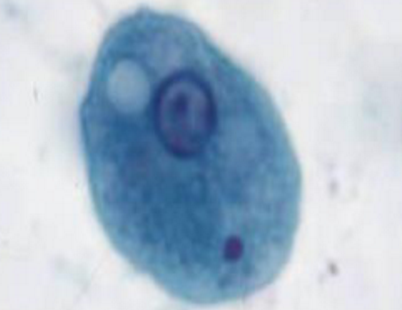
entamoeba coli trophozoite
Sluggish, non-directional motility via pseudopods
Largest amoeba—up to 50u (20-25u)
Coarse, irregular (lumpy), chromatin ring
Eccentric, irregular karyosome
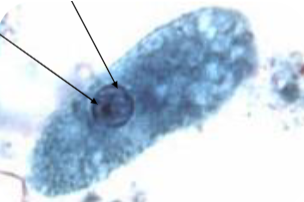
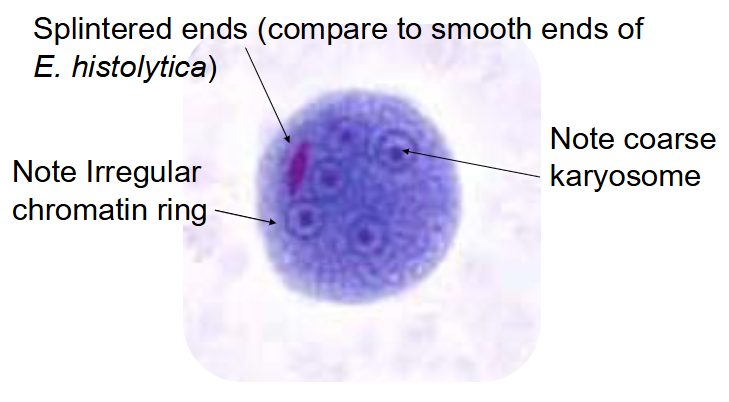
entamoeba coli cyst
Largest amoeba cyst
Up to 35u (15-25u)
Easily seen on low power (10x)
5 or more nuclei
Coarse chromatin ring
Eccentric, irregular, karyosome
Rare chromatoid bars
Splintered ends
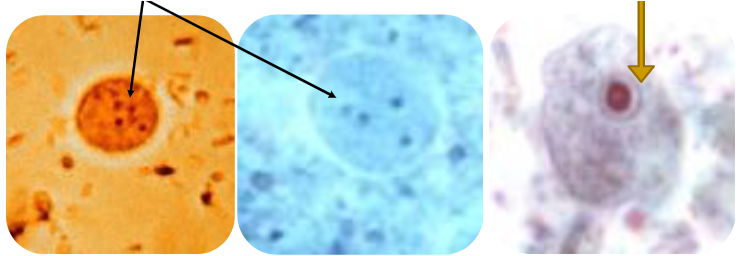
(entamoeba coli) chromatoid bar—pic
entamoeba polecki
Commensal, non-pathogenic
Usually infects pigs and monkeys
Often under diagnosed
Morphology is a mix of E histolytica and E coli
Difficult to identify unless both cysts and trophs are present on a permanent smear
Limited to Papua New Guinea, but spreading thru SE Asia
Won't be able to visually identify this on tests or in lab
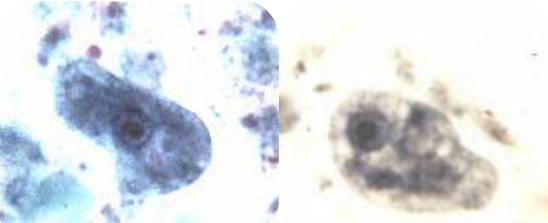
entamoeba polecki trophozoite
Large nucleus—up to 1/3 of the size of the cyst
Pleomorphic karyosome (small/large, compact/diffuse, central/eccentric)
Peripheral chromatin, evenly distributed but can be light or heavy
Chromatoid bodies highly variable in size and shape
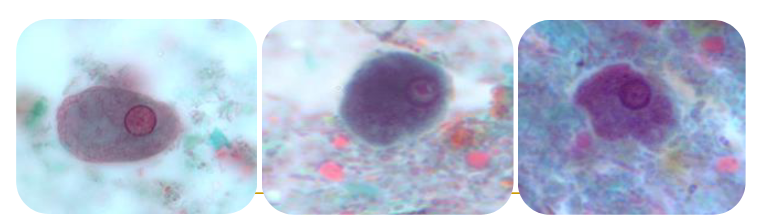
entamoeba polecki cyst
(amoeba) endolimax nana trophozoites & cyst
Troph has large dense karyosome with thin nuclear membrane—"ball and socket"
far right image ; 8-10 um
Fine granular cytoplasm
Cyst nuclei looks like potato with eyes
5-10 um (usually 6-8)
Smallest intestinal amoeba
"blot karyosome"
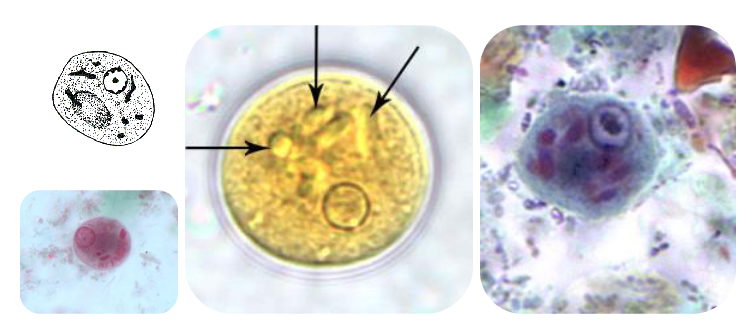
(amoeba) iodamoeba bütschlii trophozoite
Large dense karyosome
Heavier chromatin (nuclear membrane) than Endolimax nana
"dirty" cytoplasm
12-15u
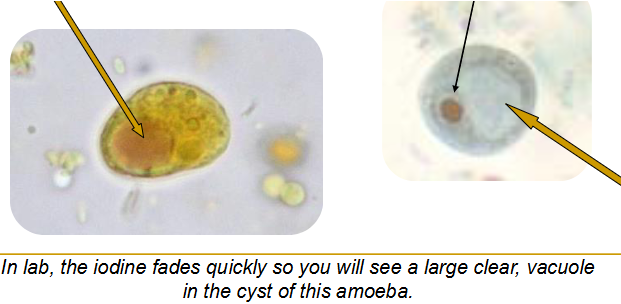
(amoeba) iodamoeba butschlii cyst
Single nucleus with large & dense karyosome; 10-12 um
Crescent halo around karyosome
Glycogen vacuole ; shimmers at you on wet mount
diagnostic procedures for amoebas
Visual examination of feces using wet and permanent mounts
6 specimens should be submitted
Immunological methods for E histolytica
Lateral flow EIA--requires non-preserved stool
Serum antibody detection: 85% pos in intestinal amoebiasis; 99% pos in extra intestinal cases
Molecular methods—PCR
(amoeba sort of) blastocystis sp (hominis)
Ubiquitous, worldwide distribution
We are still learning about this organism
Questionable parasite
Has been considered a yeast, flagellate, and an algae
Now thought to be most closely related to amoebas
Seven different subtypes, having different reservoir hosts
Has pathogenic potential
Found in up to 25% of healthy people
risk factors for acquiring blastocystis sp
Immunocompromised health
Poor hygiene practices
Be from a developing tropical country
Travel to a developing tropical country
Close contact / Exposure to animals
Consumption of contaminated food & water
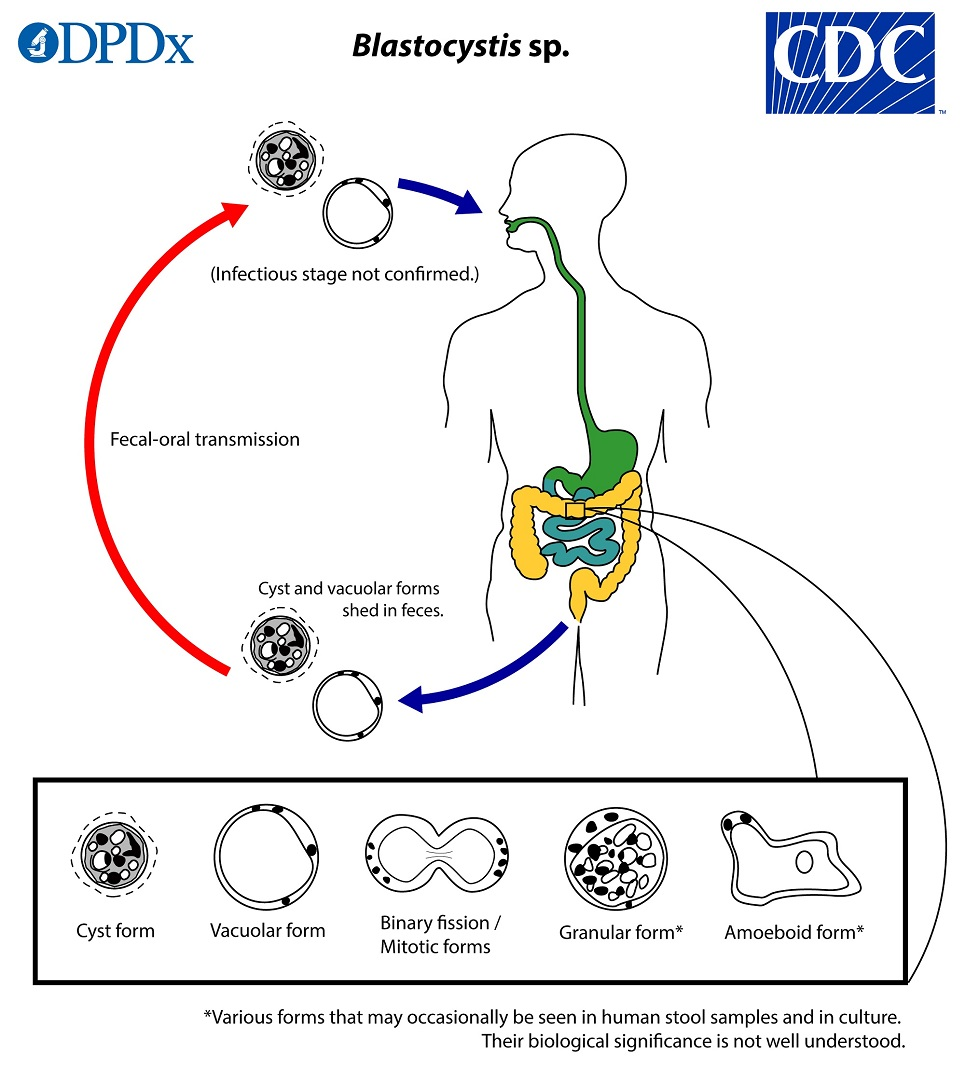
(amoeba) blastocystis life cycle
Cyst is ingested
Excystation in the large intestine
Develops into the vacuolar & other forms
Encystation during passage thru large intestine
Unknown as to cause of transition between forms
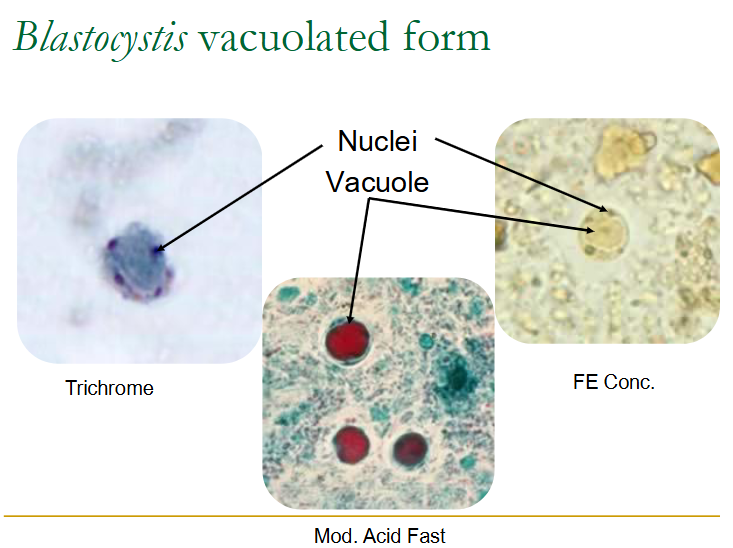
(blastocystis) vacoluated / central body form
Most common form
Form seen most often in stool specimens
Enormous variety in size
Average 5-40u (but larger ones have been seen)
Vacuole occupies 90% of the volume
Nuclei & organelles pushed to periphery
Vacuole may be ‘empty’ or have fine to flocculent material inside
(blastocystis) granular / amoeboid form
Granular form
Resembles vacuolated form
Granules throughout the organism
Amoeboid form
Rarely seen
(blastocytsis) cyst form
3-5 um (makes it very hard to find)
Easily confused with fecal debris
Environmental form
Transmissible form
blastocystis disease
Considered to be an opportunistic pathogen
Generally self-limiting
Non-specific symptoms
Abdominal pain, bloating, acute or chronic diarrhea, flatulence, nausea, anorexia
Associated with IBD (Inflammatory Bowel Disease)
diagnosis of blastocystis infections
Difficult to find and identify in stool samples
FE concentrate procedure, and permanent stains work well
Avoid washing sediment in water as this destroys the amoeboid form
Not really a problem since they are hard to detect
No fecal leukocytes present
list of flagellates (6)
Pathogenic
Dientamoeba fragilis
Giardia lamblia/intestinalis
Trichomonas vaginalis
Non-pathogenic
Trichomonas hominis
Trichomonas tenax
Chilomastix mesnili
life cycle of flagellates (general)
all flagelletes have the same life cycle; all divide by binary fission
cysts & trophs are passed in stool ; trophs do not survive in the environment
mature cysts are ingested from contaminated water or hands/fomites
cyst develops into troph in small intestine
troph divides by binary fission
troph releases cyst to repeat cycle
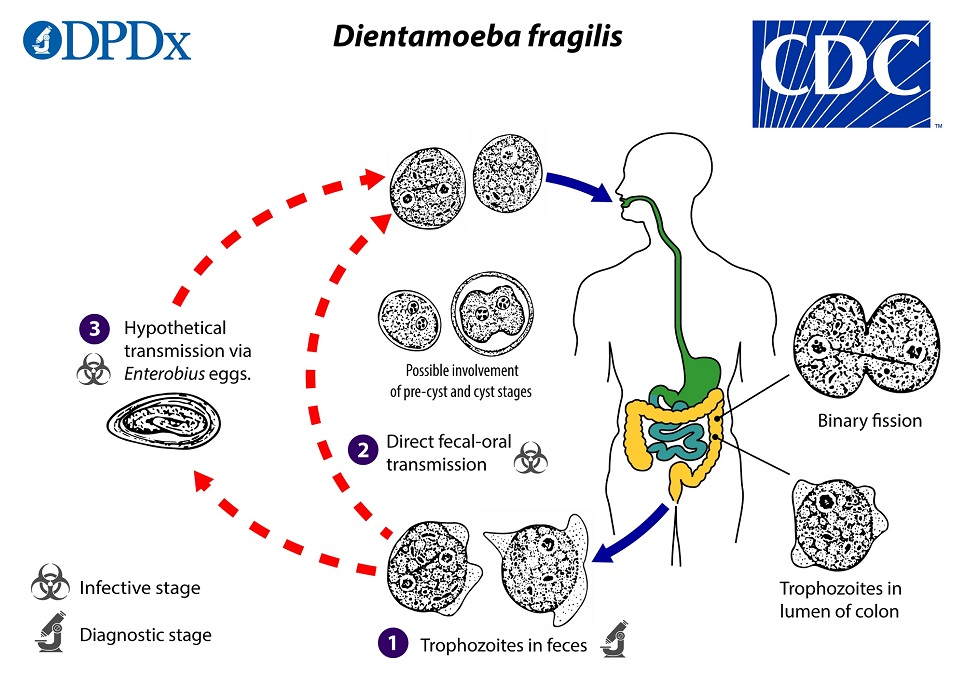
(flagellate) dientamoeba fragilis
Worldwide distribution
Occasionally pathogenic
Morphologically similar to amoebas; no external flagellum
Symptoms: colicky pains, fatigue, weight loss
Transmission unknown
Just discovered a cyst stage
LIKES TO COINFECT WITH PINWORM!
(flagellate) dientamoeba fragilis life cycle
trophozoites are found in the lumen of the large intestine, where they multiply via binary fission, and are shed in the stool
whether and in what settings transmission to humans occurs via ingestion of such forms in contrast or in addition to other fecal-oral transmission routes is not yet known
transmission via helminth eggs (e.g., via Enterobius vermicularis eggs) has been postulated
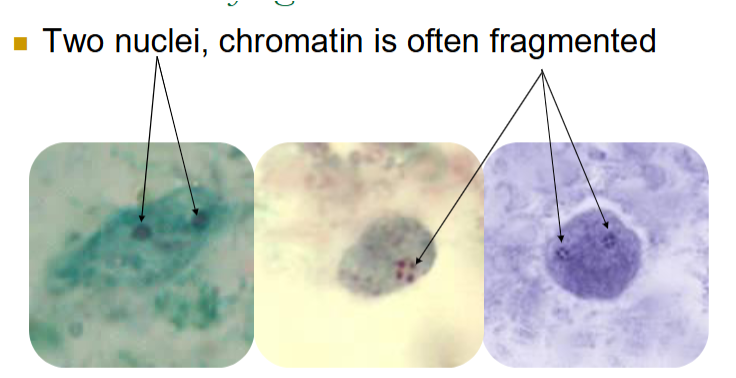
dientamoeba fragilis morphological characteristics (troph)
Size varies: 4-12; 5-15 um
20-40% of trophs have one nucleus
Karyosome often fragmented into 3-5 granules
No peripheral chromatin around nucleus
Cytoplasm is ‘dirty’
May see vacuoles and granules
Will not be seen on wet preps or in concentrates
(flagellates) giardia lamblia / intestinalis / duodenalis
Worldwide distribution; most common parasite in USA
Pathogenic
Beavers and other animals are reservoirs
Seen in campers and those who drink untreated water, kids in daycare, homosexual men
12 to 20-day incubation period
Infects upper small intestine, but does not invade tissues
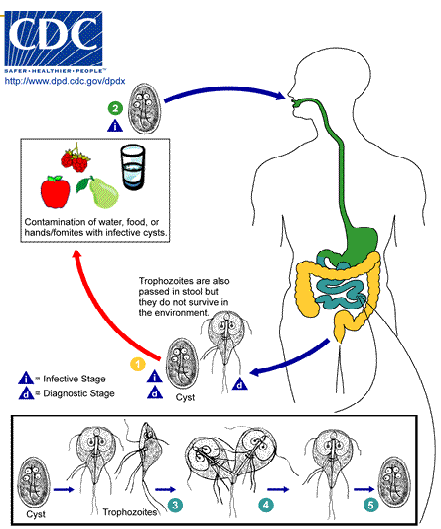
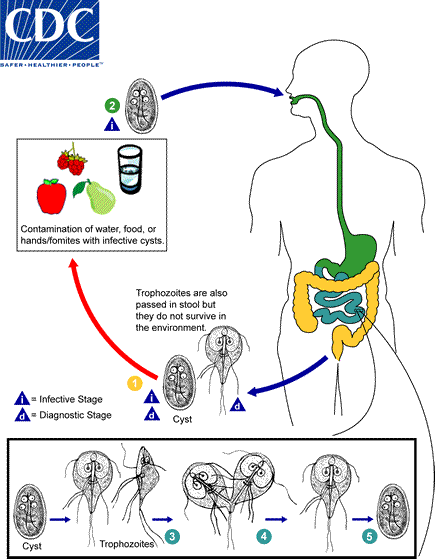
giardia lamblia / intestinalis / duodenalis life cycle
Infection occurs by the ingestion of cysts in contaminated water, food, or by the fecal-oral route (hands or fomites)
both cysts and trophozoites can be found in the feces (diagnostic stages)
In the small intestine, excystation releases trophozoites (each cyst produces two trophozoites)
Trophozoites multiply by longitudinal binary fission, remaining free in the lumen of the proximal small bowel or attached to the mucosa by a ventral sucking disk
Encystation occurs as the parasites transit toward the colon—cyst is the stage found most commonly in nondiarrheal feces
symptoms of giardia infection
Acute phase
resembles food poisoning, traveler’s diarrhea, viral enteritis, etc
Lasts only a few days
Flatulence, mushy foul-smelling stools, explosive watery diarrhea, gray green color, greasy looking, nausea, cramps, malaise, abdominal swelling
Chronic phase – recurrent, brief episodes of loose, foul-smelling, grayish, foamy stools
Abdominal discomfort & marked distention with belching
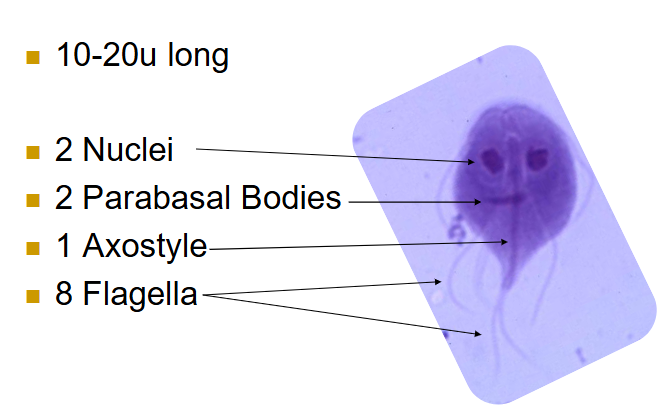
(flagellates) giardia trophozoites
10-20u
2 nuclei
2 parabasal bodies
1 axostyle
8 flagella
Falling leaf motility
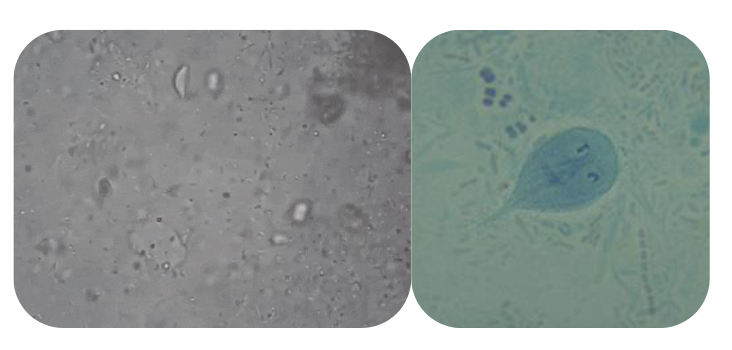
(flagellates) giardia cysts
Same features as troph, but may have 4 nuclei
Often stain faintly, can be hard to pick out from background
Size 11-14u long
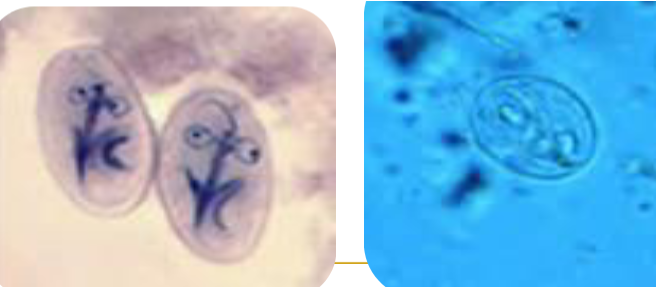
diagnostic criteria for giardia
Demonstrate cysts or trophs in stool samples
FE concentrate and Trichrome permanent smear
Demonstrate trophs in the duodenal contents
String test or duodenal biospy
Irregular shedding pattern
Collect a minimum of 3 stools on non-consecutive days
Rapid EIA procedures available
Often combined with Cryptosporidium
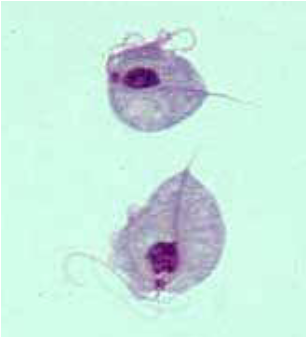
(flagellates) trichomonas vaginalis
Urogenital pathogen in men and women
Only seen in troph stage
Found in various body sites
Urine, vaginal secretions, prostatic secretions
Transmitted through sexual contact
Women – itching, burning, dysuria, foamy, yellow-green discharge, foul odor
Men – usually asymptomatic
May see prostatitis, urethritis, epididymitis, urethral stricture
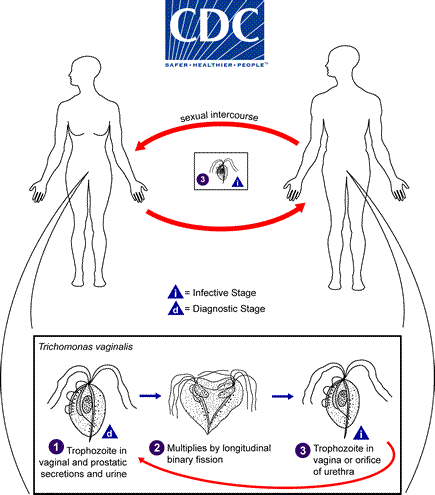
trichomonas vaginalis life cycle
Trichomonas vaginalis resides in the female lower genital tract and the male urethra and prostate where it replicates by binary fission
does not appear to have a cyst form, and does not survive well in the external environment
Trichomonas vaginalis is transmitted among humans, its only known host, primarily by sexual intercourse
(flagellates) other trichomonads
Trichomonas hominis
Non-pathogenic; no cyst
Inhabits the colon
Difficult to see and recognize in permanent stains
Look motile trophs in wet preps
Trichomonas tenax
Non-pathogenic; inhabits the mouth
Habitat determines species
(flagellates) chilomastix mesnili
Non-pathogenic
Lives in cecum & colon
Trophs have rotating, wobbling motion
chilomastix mesnili life cycle
Infection occurs by the ingestion of cysts in contaminated water, food, or by the fecal-oral route (hands or fomites)
Both cysts and trophozoites can be found in the feces (diagnostic stages)
In the large intestine, excystation releases trophozoites
Trophs reproduce by binary fission
Chilomastix resides in the cecum and/or colon
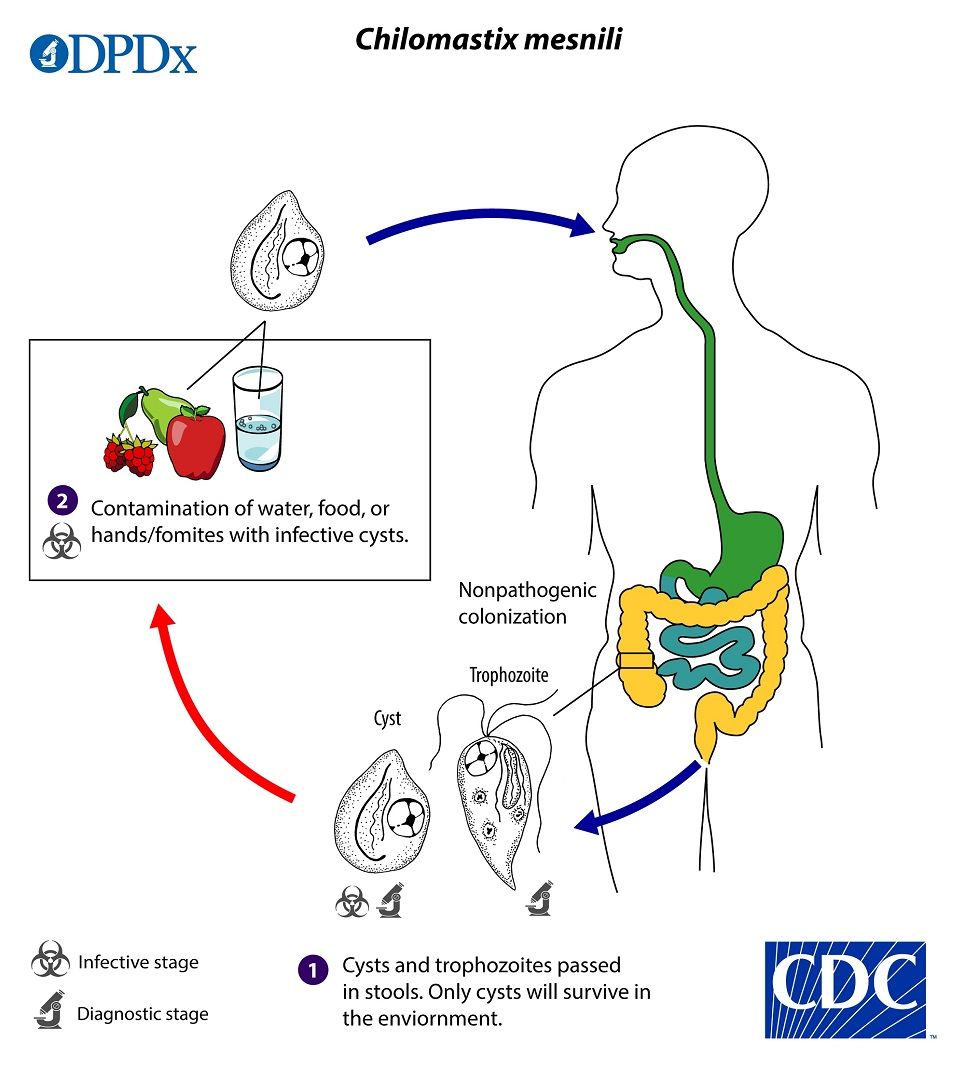
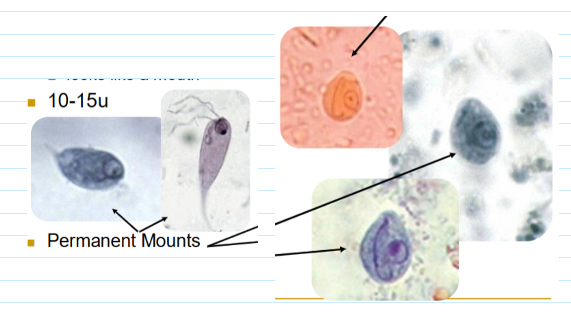
chilomastix mesnili trophozoites & cysts
Tear drop shaped troph
Eccentric nucleus usually visible
Looks like a mouth
10-15u
Lemon shaped cyst (6-11u)
only known ciliate to infect humans?
balantidium coli
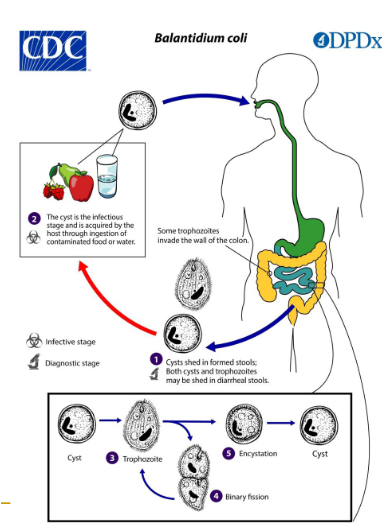
(ciliates) balantidium coli
Pathogenic
Largest protozoan parasitizing man
Lives in Colon & cecum
Worldwide distribution
High endemic areas: Latin America, Philippines, Papua New Guinea, West Iran ; Rare in USA
Close association with exposures to live pigs, esp when sanitary conditions are inadequate
Poor quality drinking water
Acquired by ingestion of food or water contaminated by fecal material containing cysts
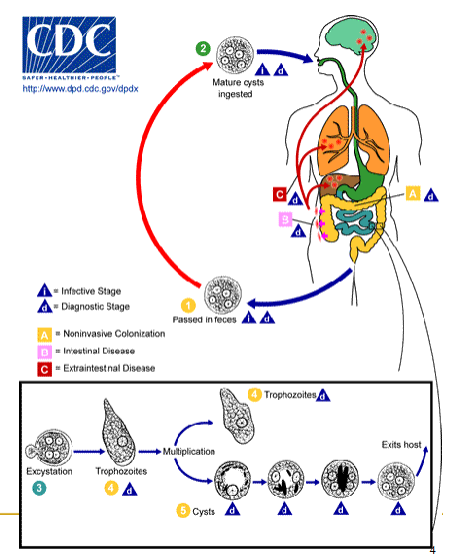
(ciliates) balantiium coli life cycle
Human ingests cysts thru contaminated food/water
Trophs can’t survive the low pH of stomach
Excystment followed by maturation
Symptoms appear on average 6 days later
Encystment in the colon and rectum
Cysts passed in formed feces
risk factors for balantidium coli
Immunocompromised
Alcoholism
Malnourishment
clinical presentations of balantidium coli (4)
asymptomatic
chronic
fulminating balantidiosis
extraintestinal infection
(balantidium coli) asymptomatic hosts / extraintestinal infection
Asymptomatic hosts
Reservoir of infection
Extraintestinal Infections
Limited mainly to the appendix
(balantidium coli) chronic infection symptoms
non-bloody diarrhea, cramps, halitosis, nausea, vomiting, tenesmus, abdominal pain
Symptoms are similar to that of Entamoeba histolytica
(balantidium coli) fulminating balantidiosis
Mucoid, bloody stools
Weight loss
Explosive diarrhea
Ulceration of the mucosa due to hyaluronidase
Flask-shaped lesions may form
Perforation of the colon may occur
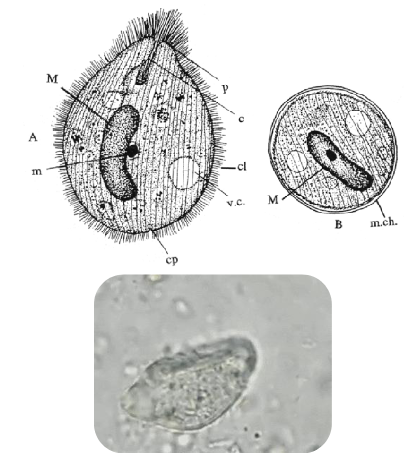
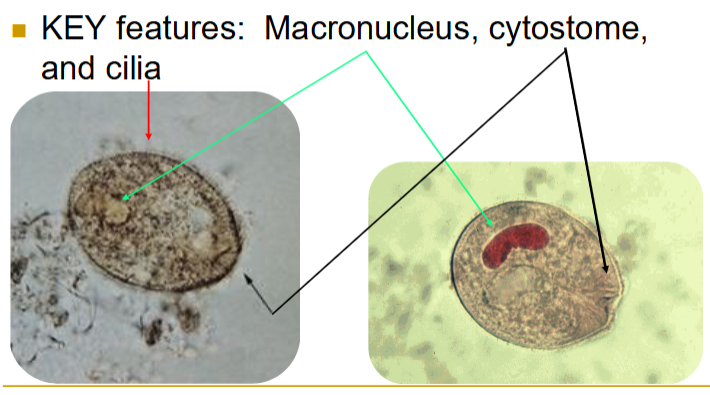
balantidium coli trophozoite
50-100u
key features: macronucleus, cytostome, and cilia
also has a micronucleus; very hard to find
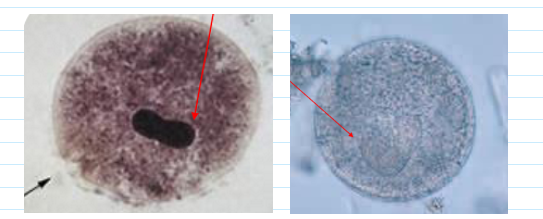
balantidium coli cyst
50-70, round with distinct cyst wall
Macronucleus; no cilia
Cytotsome—some have this
usually very circular; almost a perfect circle
list of coccidia (5)
Cystoisospora (Isospora) belli
Cryptosporidium parvum
Cyclospora
Sarcocystis
Microsporidia
coccidia (general)
Obligate tissue parasites
Inhabit mucosa of small intestine
Developmental stages resemble malaria
(coccidia) cystoisospora (isospora) belli
Causes severe intestinal disease
Infects small intestine
Diarrhea, nausea, fever, steatorrhea, headache, weight loss
Big problem in HIV Positive patients
May produce a toxin
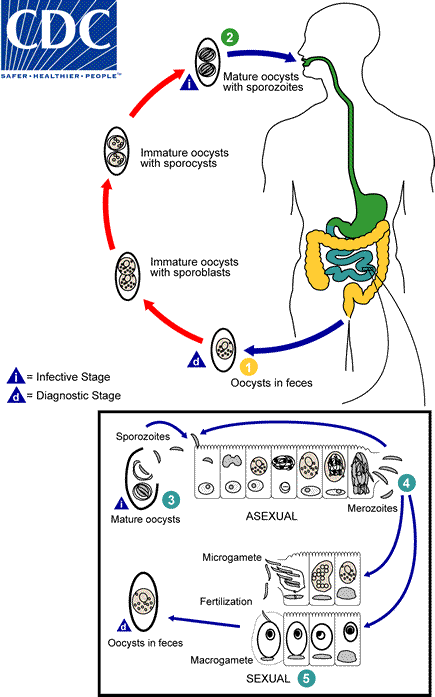
cystoisospora (isospora) belli life cycle
At time of excretion, immature oocyst contains one sporoblast
after excretion, sporoblast divides in two (oocyst now has two sporoblasts)
sporoblasts secrete cyst wall → sporocysts
sporocysts divide twice to produce four sporozoites each
Infection occurs by ingestion of sporocysts-containing oocysts: the sporocysts excyst in the small intestine and release their sporozoites, which invade the epithelial cells and initiate schizogony
Upon rupture of the schizonts, the merozoites are released, invade new epithelial cells, and continue the cycle of asexual multiplication
Trophozoites develop into schizonts which contain multiple merozoites
After a minimum of one week, the sexual stage begins with the development of male and female gametocytes
Fertilization results in the development of oocysts that are excreted in the stool
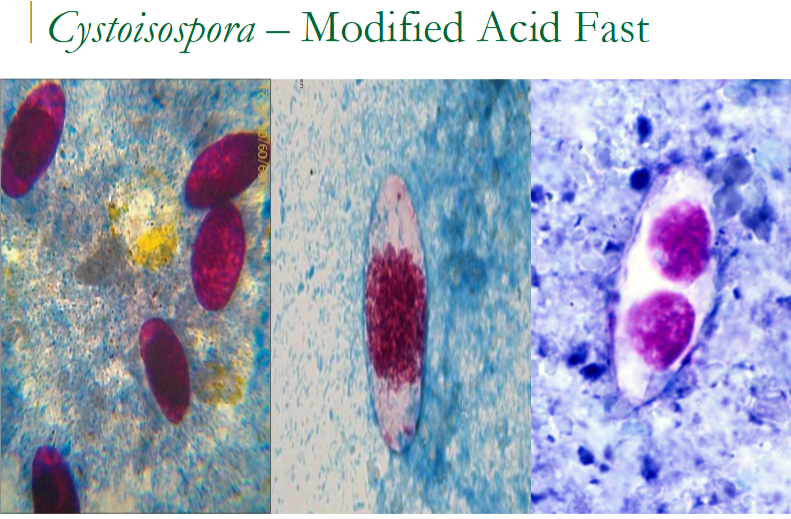
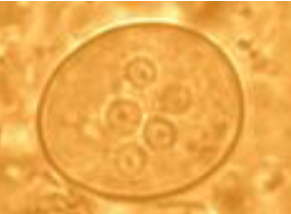
cystoisospora (isospora) belli oocyst
Immature stage contains 1 sporoblast
Mature stage contains 2 sporocysts
Each contains four sporozoites
find in wet mount (float above the sediment—focus microscope a little higher up) OR stain w modified acid fast
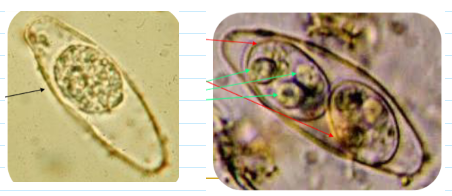
(coccidia) cryptosporidium parvum
Infects brush border of columnar epithelial cells of the small intestine
main reservoir is cattle
If you have an intact immune system:
Profuse, watery diarrhea, mild cramps, nausea, anorexia
Lasts 10-15 days, self-cure
If you have a compromised immune system
Same symptoms, but symptoms more severe
Becomes a chronic infection, lasting years
Develop extraintestinal infections
transmission of cryptosporidium parvum
occurs mainly through ingestion of fecally contaminated water (e.g., drinking or recreational water) or food (e.g., raw milk) or following direct contact with infected animals or people
oocysts are infectious upon excretion
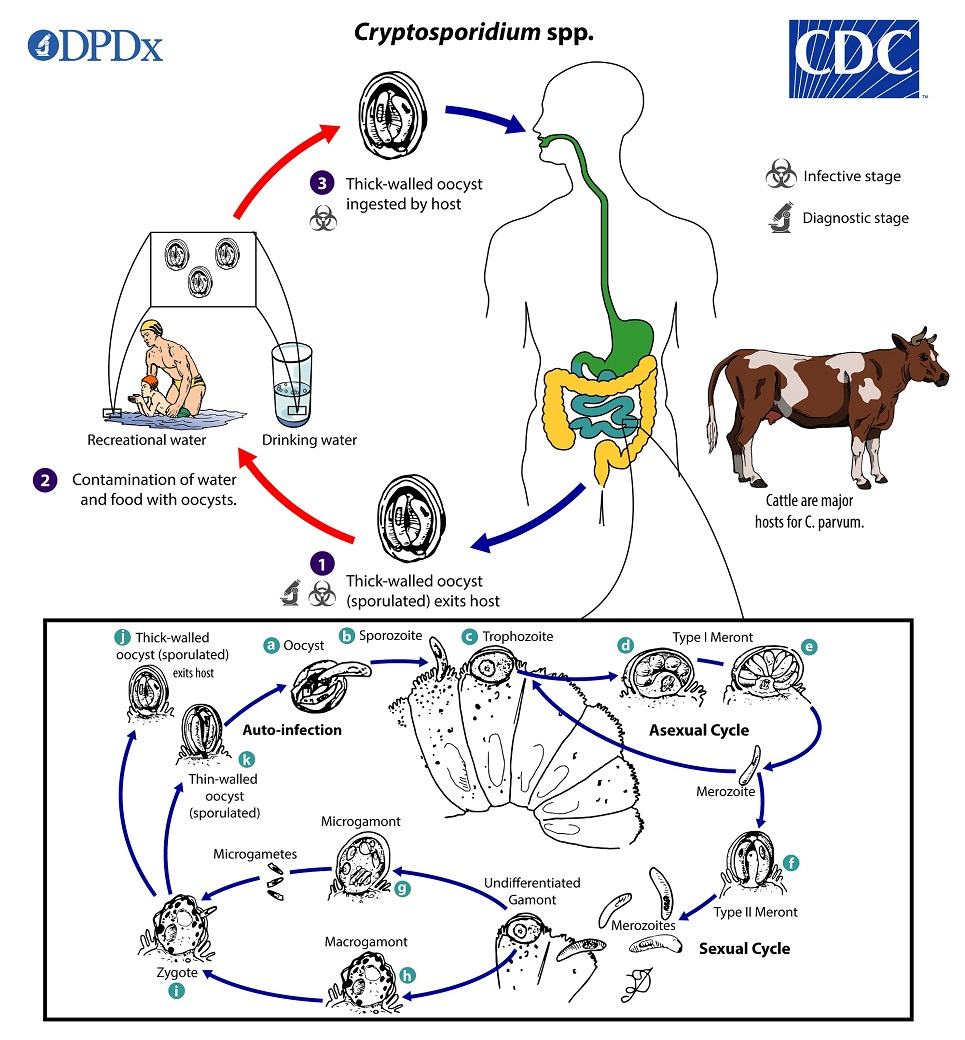
cryptosporidium parvum life cycle
sporulated oocysts, w 4 sporozoites, excreted by host in feces
excystation occurs—sporozoites are released and parasitize the epithelial cells of the gastrointestinal tract
within the brush border, the parasites undergo asexual multiplication (schizogony/merogony) and then sexual multiplication (gametogony) producing microgamonts (male) and macrogamonts (female)
Upon fertilization of the macrogamonts by the microgametes oocysts develop and sporulate in the infected host
zygotes give rise to thick- and thin-walled oocysts
thick-walled oocysts excreted from the host into the environment ; thin-walled oocysts involved in internal autoinfective cycle and are not recovered from stools
oocysts are infectious upon excretion, enabling direct and immediate fecal-oral transmission
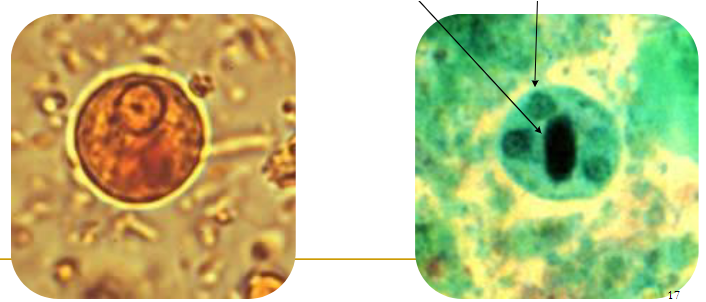
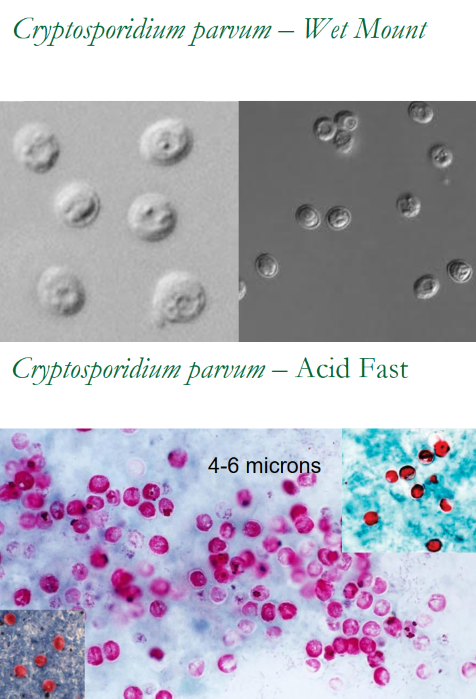
(coccidia) cryptosporidium parvum wet mount vs acid fast (pic)
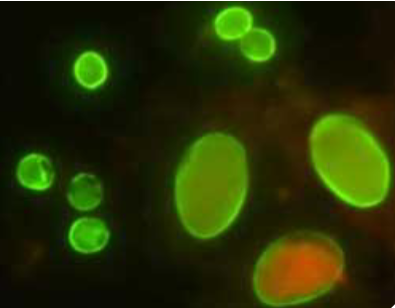
(coccidia testing) direct fluorescent antigen test
used for giardia and cryptosporidium
Performed on FE concentrate sediment
Prone to false positives
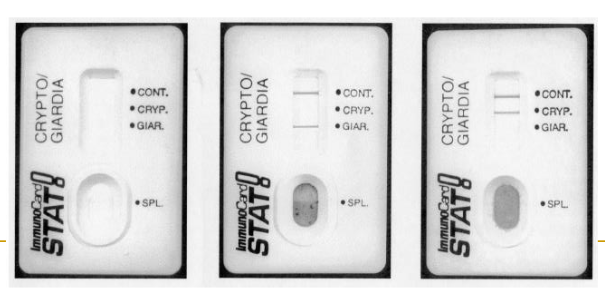
(coccidia testing) immunocard STAT
used for cryptosporidium and giardia
Lateral flow EIA test
Antibodies embedded in membrane
Performed on unconcentrated preserved feces
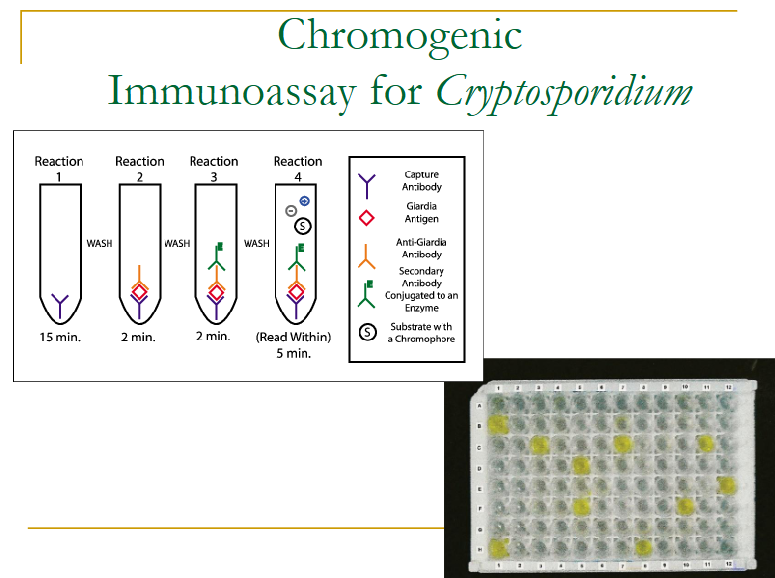
(coccidia testing) chromogenic immunoassay
only used for Cryptosporidium
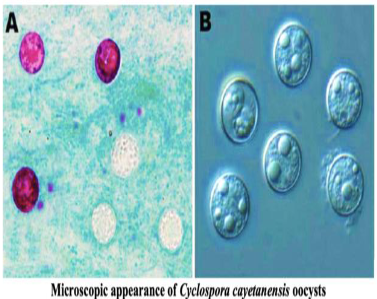
(coccidia) cyclospora
8-10 microns
Emerging pathogen
Flu-like illness with nausea, vomiting, weight loss, explosive diarrhea
Lasts 1-3 weeks
No animal reservoir
Human feces contaminated food and water
2-days to 2-weeks incubation period
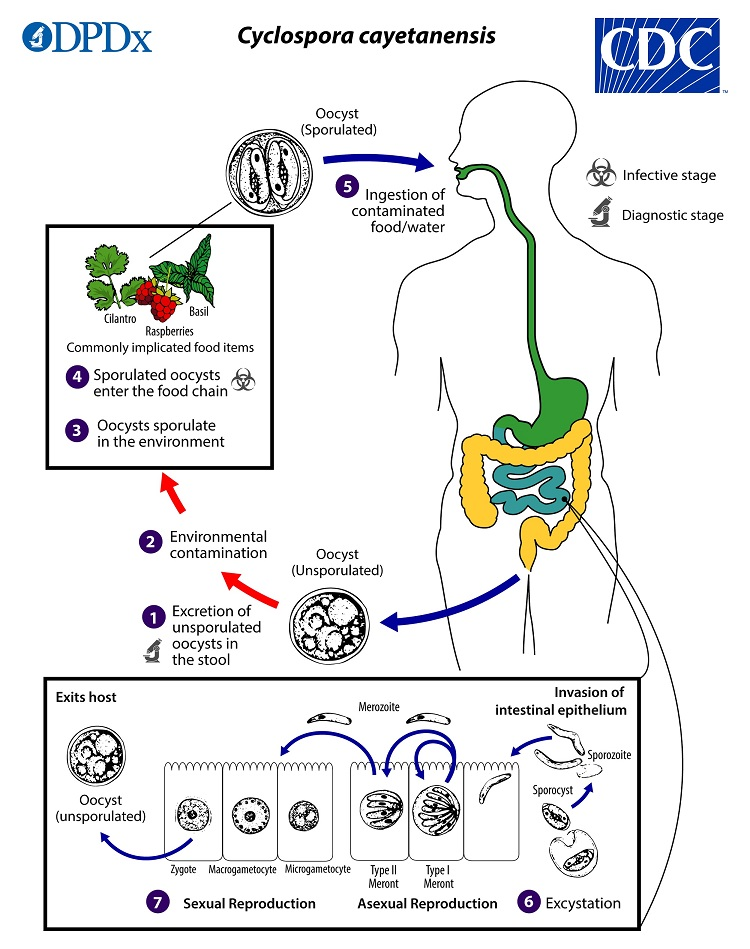
(coccidia) cyclospora life cycle
oocysts passed in stool sporulate in environment (no fecal oral transmission possible)
sporulation occurs after days/weeks at temps between 22°C to 32°C = division of the sporont into two sporocysts, each w 2 elongate sporozoites
sporulated oocysts can contaminate fresh produce (basil, cilantro, raspberries) and water which are then ingested
oocysts excyst in the gastrointestinal tract, freeing the sporozoites, which invade the epithelial cells of the small intestine
inside the cells, they undergo asexual multiplication into type I and type II meronts
merozoites from type I meronts likely remain in the asexual cycle
merozoites from type II meronts undergo sexual development into macrogametocytes and microgametocytes upon invasion of another host cell
fertilization occurs, and the zygote develops to an oocyst which is released from the host cell and shed in the stool
diagnosis of cyclosporiasis
requires submission of stool specimens for 'Ova and Parasite' testing with additional specific orders for Cyclospora identification
A single negative stool specimen does not exclude the diagnosis
3 specimens are optimal
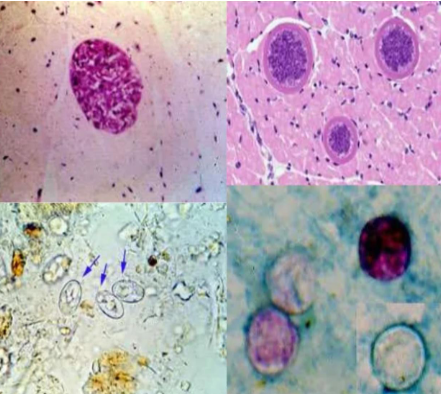
(coccidia) sarcocystis
9-16 microns
Infects various mammals
Human: Definitive Host
Pig: Intermediate Host
Acquired from improperly cooked or raw beef or pork
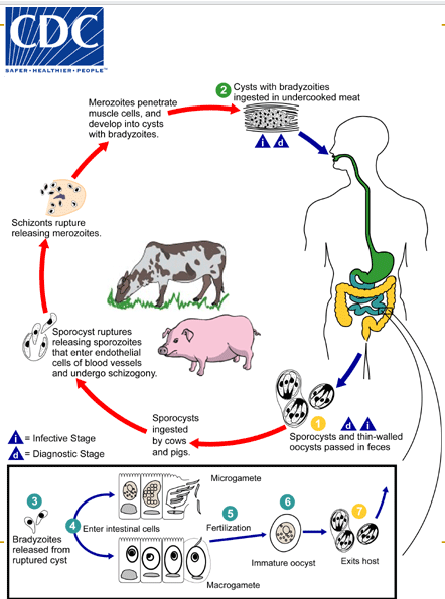
(coccidia) sarcocysitis life cycle
sporulated oocysts (containing 2 sporocysts) and individual sporocysts passed in stool
Sporocysts ingested by cattle/pigs & rupture, releasing sporozoites
Sporozoites enter endothelial cells of blood vessels and undergo schizogony → first-gen schizonts
Merozoites from first-gen invade capillaries and blood vessels, becoming 2nd-gen schizonts
2nd gen merozoites invade muscle cells and develop into sarcocysts containing bradyzoites = infective stage for the definitive host
Humans become infected by eating undercooked meat containing sarcocysts; bradyzoites released from ruptured cysts in small intestine and invade lamina propria of the intestinal epithelium
There, they differentiate into macro- and microgametocytes. Fusion of male and female gametes results in the formation of oocysts
Oocysts sporulate in the intestinal epithelium and are shed from the host in feces
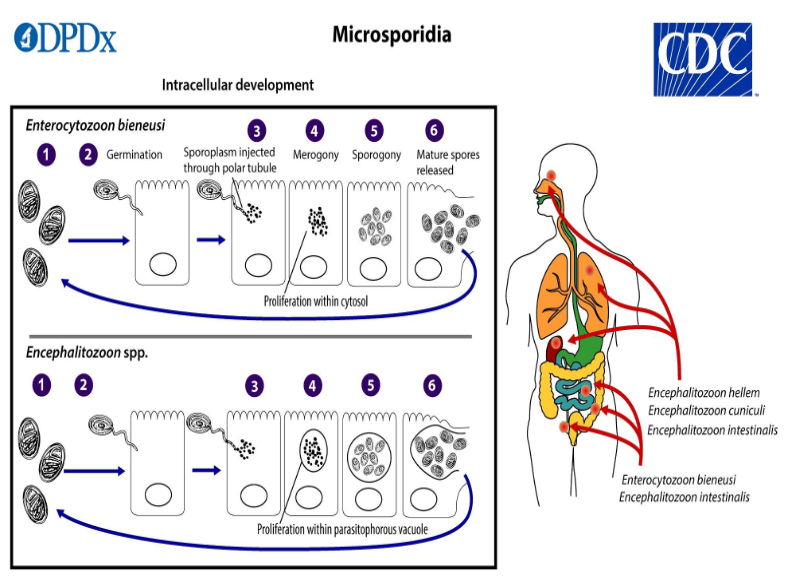
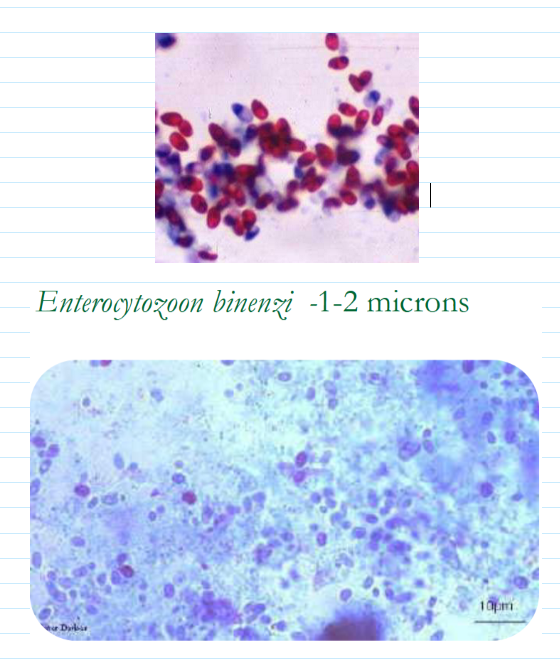
(coccidia) microsporidia
1.5-2 microns
Obligate intracellular parasite
Only one species associated with human disease
Frequently found in HIV Positive patients
Impossible to find in feces
Biopsies are best specimen for diagnosis
(coccidia) microsporidia life cycle
Infective spore germinates, rapidly everting its polar tubule which contacts the eukaryotic host cell membrane
The spore injects the sporoplasm into the host cell through the polar tubule
sporoplasm enters the proliferative phase marked by extensive multiplication, creating meronts thru binary/ multiple fission
meronts undergo sporogony creating sporonts and eventually mature spores when all organelles are polarized
when spores increase & completely fill the host cell cytoplasm, the cell membrane is disrupted and spores are released to the surroundings
free mature spores can infect new cells thus continuing the cycle