MCAT General Chemistry - Acids and Bases
1/50
Earn XP
Description and Tags
586
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
51 Terms
cocaine production
treat coca leaves with acid → cocaine: snorted or injected, water soluble
treat coca leaves with base → freebase/crack: smoked and inhaled, water insoluble
Arrhenius acid
dissociate to form an excess of H+ in solution; limited to aqueous acids and bases
contain H at the beginning of their formula
ex. HCl, HNO3, H2SO4
Arrhenius base
dissociate to form an excess of OH− in solution; limited to aqueous acids and bases
contain OH at the end of their formula
ex. NaOH, Ca(OH)2, Fe(OH)3
Brønsted–Lowry acid
species that donates hydrogen ions (H+); not limited to aqueous solutions; also always a Lewis acid

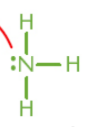
Brønsted–Lowry base
species that accepts hydrogen ions (H+); not limited to aqueous solutions; also always a Lewis base

Lewis acid
lone electron pair acceptor; not always Brønsted–Lowry acid
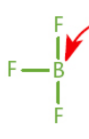
Lewis base
lone electron pair donor; not always Brønsted–Lowry base
amphoteric
reacts like an acid in a basic environment and like a base in an acidic environment
ex. water, conjugate base of a polyvalent acid, hydroxides of certain metals, amino acids that have a zwitterion intermediate
amphiprotic
can either gain or lose a proton
Acid naming
formed from anions with names that end in –ide = prefix hydro– + anion root + –ic
formed from oxyanions = prefixes and roots retained
-ite = –ous acid.
-ate = -ic acid
autoionization
water can react with itself, creating acid and base; reversible
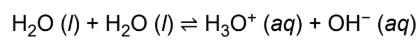
hydronium ion (H3O+)
water with proton
hydroxide ion (OH−)
water sans proton
water dissociation constant, Kw
Kw = [H3O+][OH−] = 10−14 at 25°C (298 K)
concentrations of the hydrogen ions and hydroxide ions are always equal in pure water at equilibrium
pH scale
concentration scale for acidity; negative logarithm of concentration of protons/hydronium
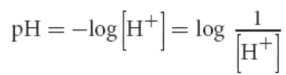
pOH scale
concentration scale for basicity; negative logarithm concentration of hydroxide
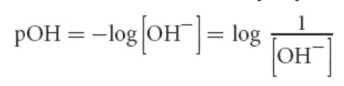
pH-pOH scale
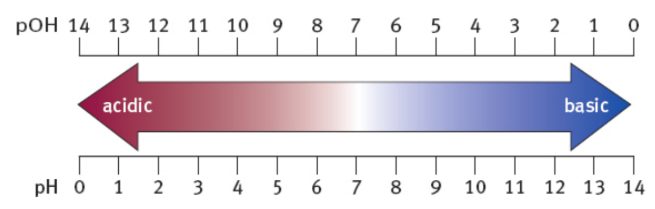
As pH increases, pOH decreases by the same amount
pH < 7 = pOH > 7 = relative excess of protons = acidic
pH > 7 = pOH < 7 = relative excess of hydroxide = basic
approximation of a p scale
if the nonlogarithmic value is written in proper scientific notation, it will be in the form n × 10−m, where n is a number between 1 and 10
p value ≈ m − 0.n
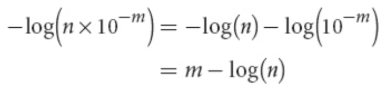
Strong acids and bases
completely dissociate into their component ions in aqueous solutions
HI, HBr, HCl, HClO4, HNO3, H2SO4
NaOH, KOH, etc.
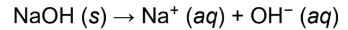
weak acids and bases
only partially dissociate in aqueous solutions to achieve an equilibrium state
Ka/Kb < 1.0
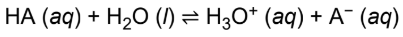
acid dissociation constant (Ka)
smaller the Ka, the weaker the acid, and consequently, the less it will dissociate
inversely related to Kb
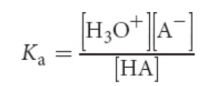
base dissociation constant (Kb)
smaller the Kb, the weaker the base, and consequently, the less it will dissociate
inversely related to Ka
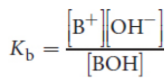
conjugate acid
acid formed when a base gains a proton
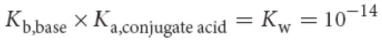
conjugate base
base formed when an acid loses a proton
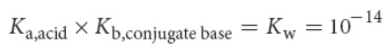
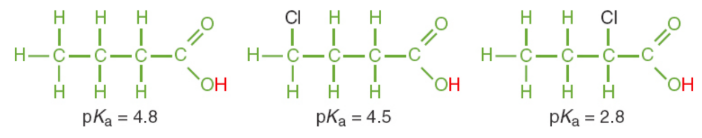
induction
Electronegative elements positioned near an acidic proton increase acid strength by pulling electron density out of the bond holding the acidic proton, weakening proton bonding and facilitating dissociation
neutralization reaction
Acids and bases may react with each other to form a salt and often water
HA (aq) + BOH (aq) → BA (s) + H2O (l)
hydrolysis
salt ions react with water to give back the acid or base
Strong acid + strong base
fully turn to salt and water; neutral solution
HCl + NaOH → NaCl + H2O
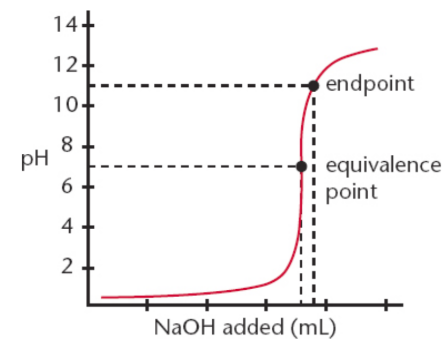
Strong acid + weak base
slightly acidic solution; usually does not make water
HCl + NH3 → NH4Cl
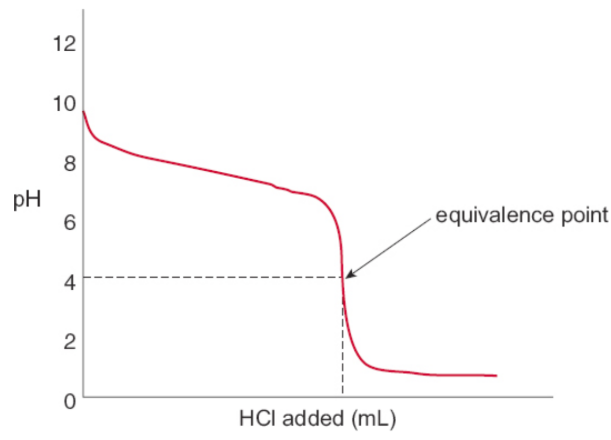
Weak acid + strong base
slightly basic solution; usually does not make water
HClO + NaOH → NaClO + H2O
Weak acid + weak base
final pH depends on the relative strengths of the reactants
HClO + NH3 → NH4ClO
Peptide Bond Formation
An acidic carboxyl group reacts with a basic amino group
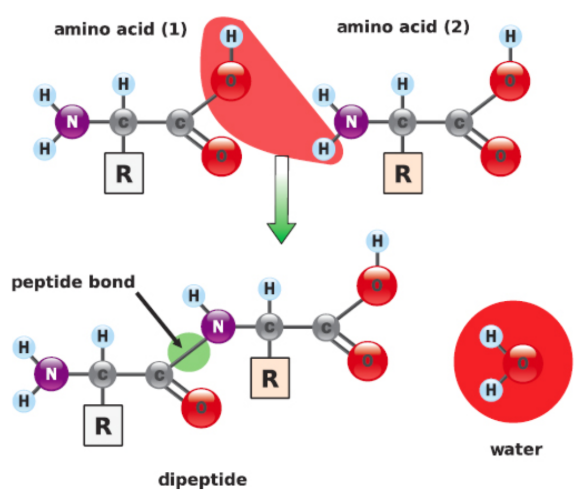
acid equivalent
equal to one mole of H+/H3O+ ions
base equivalent
equal to one mole of OH− ions
polyvalent (polyprotic)
each mole of the (Brønsted-Lowry) acid or base liberates more than one acid or base equivalent
normality
quantity of acidic or basic capacity
ex. each mole of H3PO4 yields three equivalents of H3O+ → 2 M H3PO4 = 6 N
gram equivalent weight
the mass of a compound that produces one equivalent (one mole of charge)
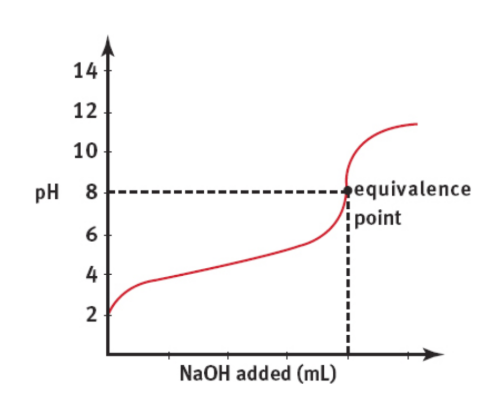
Titration
procedure used to determine the concentration of a known reactant in a solution; adding small volumes of the titrant to a known volume of a solution of the titrand until completion of the reaction is achieved
titrant
solution of known concentration used to identify the pH of the titrand
titrand
a solution of unknown concentration to which the titrant is added
equivalence point
the number of acid equivalents present in the original solution equals the number of base equivalents added, or vice-versa
only 7 for strong acid-base due to full dissociation
NaVa = NbVb
where Na and Nb are the acid and base normalities and Va and Vb are the volumes of acid and base solutions
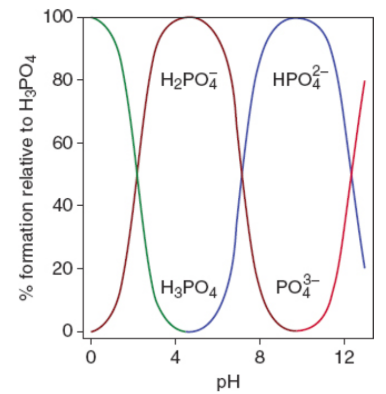
Speciation Plot
At any given pH, only two forms of the acid exist in solution; thus, each conjugate is titrated separately
pH meter
measures pH
indicator
weak organic acids or bases that have different colors in their protonated and deprotonated states; leads to a change in the absorption spectrum of the molecule; must always be a weaker acid or base than the acid or base being titrated
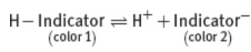
endpoint
The point at which the indicator changes to its final color; if done well, volume difference between it and the equivalence point is negligible
titration curve for a polyvalent acid or base
multiple equivalence points
ex. amino acids
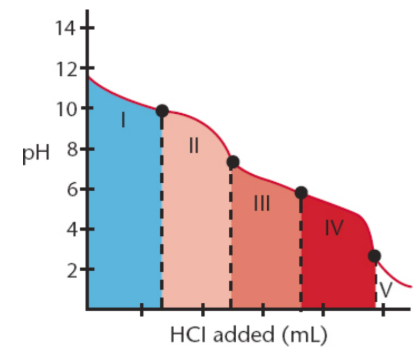
half-equivalence point
occurs when half of a given species has been protonated (or deprotonated)
buffer solution
mixture of a weak acid/base and its salt that resist change in pH; generally maintained within 1 pH unit of the pKa value
bicarbonate buffer system
the H2CO3/HCO3− conjugate pair in the plasma component of the blood for maintaining the pH of the blood within a fairly narrow physiological range
Henderson–Hasselbalch equation
used to estimate the pH/pOH of a buffer solution
pH = pKa + log [A-]/[HA]
pOH = pKb + log [B+]/[BOH]
![<p>used to estimate the pH/pOH of a buffer solution</p><p>pH = pKa + log [A-]/[HA]</p><p>pOH = pKb + log [B+]/[BOH]</p>](https://knowt-user-attachments.s3.amazonaws.com/25d8d62a-13ec-4402-8801-445fa0ba8096.png)
buffering capacity
the ability to which the system can resist changes in pH