Chemistry Unit 2 AOS 1
1/166
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
167 Terms
Water is the only substance that can naturally occur as a ___, ___ and ___.
Solid, liquid, gas
What process is occurring in each of these:
Liquid --> Gas
Liquid --> Solid
Gas --> Liquid
Solid --> Liquid
Evaporation
Freezing
Condensation
Melting
Water makes up more than __% of Earth’s surface and there is approximately 1.386 billion km3 on Earth.
70%
What type of water is potable/drinkable by humans and state why?
Freshwater
As Water needs to have very low salt content in order to be drinkable by humans
Approximately 3% of water is freshwater --> however most is trapped in ___, ___ or in ___ --> only 1% of freshwater is surface and accessible (so only 0.03% of water is both fresh + accessible)
Ice caps, glaciers, groundwater
Can humans drink water straight from lakes, rivers and springs? Why/Why not?
No
As water needs to be cleaned and distributed throughout a population using infrastructure and systems
What are group 16 hydrides? List some examples.
Compounds that consist of a group 16 element covalently bonded to 2 hydrogen atoms
e.g. water (H20), hydrogen sulfide (H2S), hydrogen selenide (H2Se), hydrogen telluride (H2Te), hydrogen polonide (H2Po)
What is the boiling point of water?
100 °C
Using this diagram, outline the pattern and state why water has the highest boiling point?
Moving down a group, the size of elements increase --> causing the dispersion forces between the molecules to increase in strength (as size of molecules increase, dispersion forces increase in strength) --> higher boiling points
However while water molecules are small due to oxygen being the smallest element in group 16 --> it has the highest boiling point in the group 16 hydrides as it can form hydrogen bonds (strong intermolecular force)
What is specific heat capacity (c)? And what unit is it measured in?
Amount of energy required (joules, J) to raise the temperature of 1 gram of a substance by 1 °C
Measured in J g−1 °C−1 (joules per gram per degree Celsius) (can also be written as kJ kg−1 °C−1 kilojoules per kilogram per degree Celsius)
What is the specific heat capacity of water?
4.18 J g−1 °C−1
Why does water have a relatively high specific heat capacity?
Water has a relatively high specific heat capacity due to the strong hydrogen bonds --> allows it to absorb a large quantity of thermal energy before increasing in temperature
What is the formula for calculating the amount of thermal energy required to raise the temperature of a given mass of a substance by a given number of degrees?
q: amount of energy (joules)
m: mass (grams)
c: specific heat capacity
ΔT: change in temperature
What is the amount of thermal energy required to raise the temperature of 100 grams of water from 20 degrees to 30 degrees?
What is the density of water at 25 degrees celsius?
Density of water at 25 °C is 1.0 mL –1
How much does 1 ml of water weigh? And hence how to convert from volume (mL) to mass (grams)?
1 mL of water weighs 1.0 g
To convert from volume (mL) to mass (grams) --> volume x1.0
Why is water denser as a liquid than as a solid?
--> this is due to when water is below its freezing point of 0 °C, each water molecule forms 4 hydrogen bonds with neighboring water molecules --> however the rigid, tetrahedral lattice structure prevents water molecules from being close together
--> However as a liquid, the random arrangement of water molecules allows strong hydrogen bonds to pull water molecules closer together --> resulting in less empty space between liquid state than in ice
What is the latent heat of vaporisation?
Amount of energy required to convert 1 mol of a substance from its liquid state to its gaseous state at the boiling point of the substance
What occurs to water during:
Before boiling
At boiling point
During boiling
Before boiling
When heated, the temperature of the liquid increases --> the particles move faster as they absorb energy
At boiling point
The temperature stays the same --> as the energy being continually absorbed is now being used to break the bonds between particles and not towards increasing temperature
During boiling
Liquid is transformed into a gas by the absorbed heat energy --> this is known as the latent heat of vaporization
What is water’s latent heat of vaporisation? And why does water have a relatively high latent heat of vaporisation?
44.0 kJ mol-1
Due to the strong hydrogen bonds in water, these strong forces are able to absorb more energy before becoming a gas
What is the formula for calculating the amount of heat energy required to boil water at 100 °C?
q: Quantity of heat energy (kJ)
n: Amount of water present (mol)
State what properties of water and how they help in each of these situations?
Allowing internal body temperature to stay constant + keeps water temperatures stable from solar energy
Allows water to not evaporate quickly and stay liquid in hot climates
Water's high heat capacity allows it to absorb + lose a lot of heat without changing temperature
It takes a lot of energy to turn water into liquid
List the properties of acids:
Corrosive
Low pH (less than 7 / 0-6)
Taste sour
Litmus paper – blue --> red
Reacts with bases
What are acids used for?
Clean metal surfaces
Car batteries, fertilisers, drugs, plastics, explosives and manufacturing
Enhance flavour + food preservation
List the properties of bases:
Also corrosive (but specifically caustic)
High pH (greater than 7 / 8-14)
Taste bitter
Litmus paper – red --> blue
Neutralises acids
Feel slippery/soapy
What are bases used for?
Cleaning products (e.g. oven cleaners, floor cleaners, soap)
State the Brønsted-Lowry theory:
Brønsted-Lowry theory: defines an acid-base reaction as the donation of a proton H+ from an acid to a base
Acids are proton doners
Bases are proton acceptors
Complete these:
The reaction between an acid and base is a _____ reaction
Proton transfer
List some examples of acids:
List some examples of bases:
What is a conjugate pair? And what order are they written in?
Two species which differ by one proton H+
--> In a conjugate pair always place the substance that has one more proton first
List the conjugate pairs in this acid-base reaction:
What is hydronium/protonated water?
H3O+
In this reaction state which substances are the base and acid:
--> Therefore, the nitric acid is a proton donor/acid
--> Water was a proton acceptor/base
In this reaction state which substances are the base and acid:
--> Therefore, the ammonia was a base/proton acceptor
--> Water was an acid/protein donor
If water reacts with an acid, ___ is produced.
If water reacts with a base, ___ is produced.
hydronium (H3O+) ion
hydroxide (OH−) ion
What are alkalis?
Group of bases that are soluble in water that form hydroxide ions
Do all acid-base reactions involve water as a reactant?
Not all acid-base reactions involve water as a reactant --> however do require water to occur --> this may occur when other reactants are in an aqueous solution with water
What are amphiprotic substances? List some examples.
Substances that can act as either an acid or base --> they can both donate and accept a proton (H+) in an acid-base reaction
What is deprotonation?
Process in which an acid gives up a proton (H+)
What are monoprotic acids?
Acids that will only donate one proton (H+)
--> once they donate their only hydrogen ion, they can no longer react as acids in acid-base reactions (e.g. nitric acid / HNO3)
What are polyprotic acids?
acids that can donate multiple protons (H+)
--> they can donate a proton and then the compound formed from the deprotonation can again donate a proton
What are diprotic acids?
acid that can donate two (H+) ions/ protons
Sulfuric acid ( H2SO4) is a diprotic acid. Showcase all the acid-base reactions when it reacts with water. Compare the first ionisation and second ionisation.
The first ionisation is very strong (100% ionisation) --> where every H₂SO₄ molecule donates one H⁺ easily
However the second ionisation is weaker (about 10% ionisation) --> partial ionisation
It has been shown that when sulfuric acid reacts with a base, its second proton is lost completely/to a greater extent compared with when it reacts with water. Why is this?
Amphiprotic substances such as water are weak bases and hence cannot pull H+ as strongly
What are triprotic acids?
Acids that can donate three H+ ions/ protons
Phosphoric acid ( H3PO4) is a triprotic acid.
Showcase all the acid-base reactions when it reacts with water.
Compare the first ionisation, second ionisation and third ionisation and explain why?
Outline what molecules would be in this solution?
First deprotonation: the proton/ H+ ion is easily donated as the molecule is neutral
Second deprotonation: due to the molecule having a negative charge --> this attracts the positive proton, making it harder for it to leave --> therefore occurs to a low extent
Third deprotonation: due to the molecule now having two negative charges --> its pulling even harder on the positive proton --> therefore occurs to a low extent
In this reaction, it would not only be PO43− and H+ ions --> it would instead be a mixture of H3PO4, H2PO4 −, HPO4 2−, PO43− and H+ ions
What does the bidirectional arrow (⇌) mean?
Show that the reaction will not go to completion and instead have a large proportion of reactants that haven’t ionised and only a small portion that has ionised + reversible (double arrow)
What does the unidirectional arrow (→) mean?
Show a complete reaction, where there are little to no reactants that haven’t ionised + not reversible (single arrow)
True or false? Explain why?
Many amphiprotic species are formed from the deprotonation of a polyprotic acid.
True.
Many amphiprotic species are formed from the deprotonation of a polyprotic acid --> as any proton that is lost can be reaccepted if the polyprotic acid acts as a base in another reaction
List some monoprotic, diprotic and tripotic acids:
What is the strength of an acid or base?
The strength of an acid or base is the measure of its ability to donate or accept a H+ ion/ proton
What is a strong acid? List some examples.
A strong acid undergoes almost complete ionisation when added to water --> where all the acid molecules will donate all their protons to form aqueous H+ ions
What is a weak acid? List some examples.
A weak acid partially ionises --> does not readily give up its H+ ions
What is a strong base? List some examples.
Strong bases readily accept a H+ ion during acid-base reaction // mostly or completely ionises in water
E.g. Strong bases
Oxide ion, O2−
Hydroxide ion, OH−
Hydrogen phosphate, HPO4 2-
What is a weak base? List some examples.
Weak bases do not readily accept H+ ions during acid-base reaction // partially ionise in water
E.g. Ammonia
Compare strength and concentration:
Strength: relative tendency to donate or accept protons (no quantitative value)
Concentration: relative amount of dissolved solute (quantitative value)
How is concentration measured?
Concentration can be measured by molarity (M) (number of moles of a solute dissolved in one litre of a solution (mol L-1)
Outline what concentration and strength these are:
What is changed when molecules are added or removed from a solution?
Adding or removing molecules from solution --> change its concentration, not strength
Strength is based on the identity of the acid or base itself
What is a redox reaction? And is it an acid reaction?
Acid + metal --> ionic salt + hydrogen gas
No it is not an acid reaction
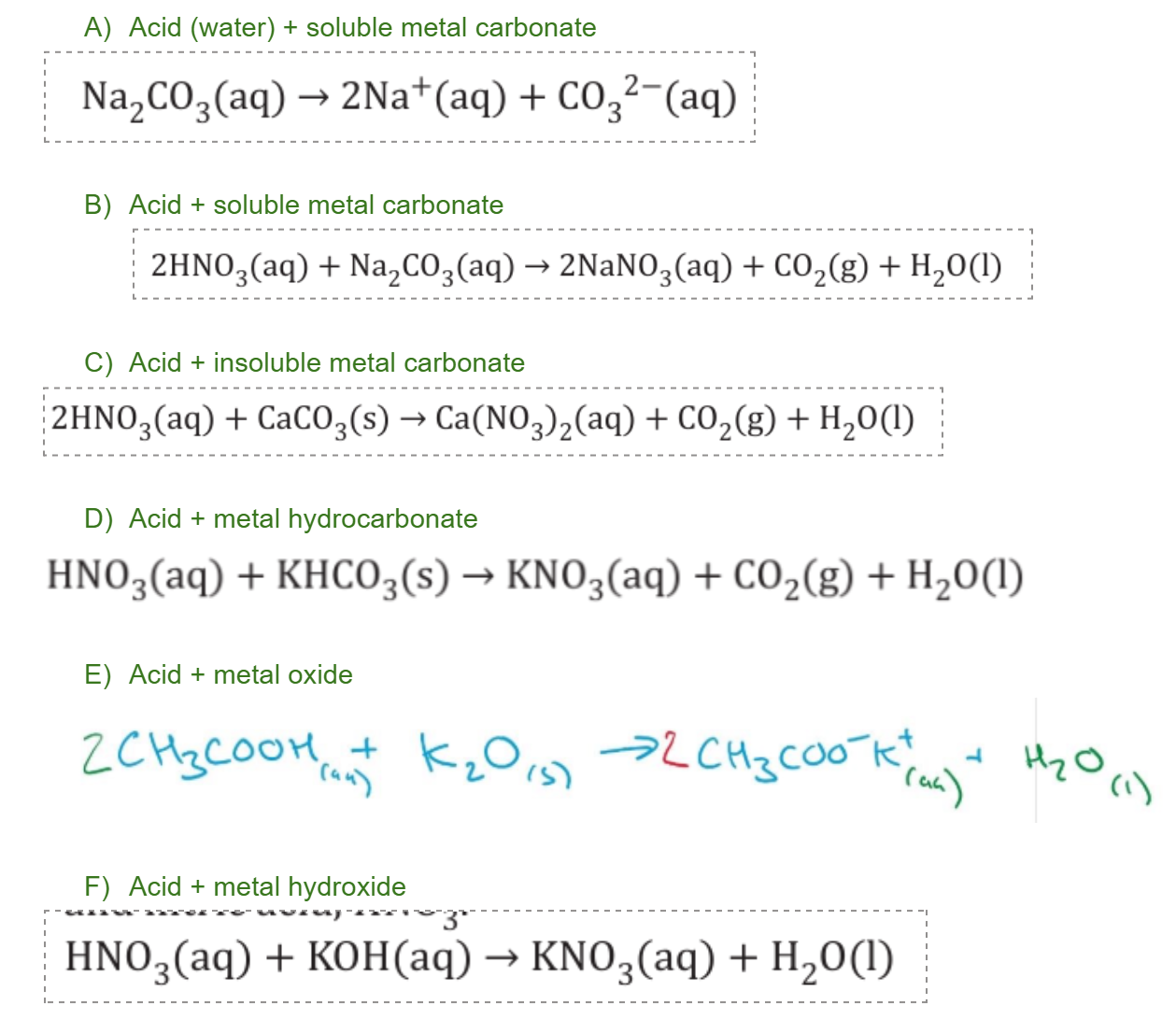
What are metal carbonates? Are they soluble or insoluble?
Metal carbonates: bases formed from metal cation and carbonate ions ( CO3 2−)
Either soluble or insoluble in water
Give some examples of soluble metal carbonates:
Sodium carbonate Na2CO3
Potassium carbonate K2CO3
When reacting with water, soluble metal carbonates will ____ to form its ___ and ___ ions.
Dissociate, cation, carbonate ions
What occurs when an acid reacts with a metal carbonate?
Find the net ionic equation of this reaction:
Give some examples of insoluble metal carbonates:
Magnesium carbonate, MgCO3
Calcium carbonate, CaCO3
Zinc carbonate, ZnCO3
What occurs when insoluble metal carbonates react with water?
Insoluble metal carbonates do not dissolve in water (stay as solids) --> do not break into ions
Find the net ionic equation of this reaction:
What are metal hydrogencarbonates/bicarbonates? List some examples.
Metal hydrogencarbonates/bicarbonates: compounds containing a metal cation and bicarbonate ion (HCO3 −)
e.g.
sodium hydrogencarbonate, NaHCO3
potassium hydrogencarbonate, KHCO3
magnesium hydrogencarbonate, Mg(HCO3)2
calcium hydrogencarbonate, Ca(HCO3)2
What occurs when an acid reacts with a metal hydrogencarbonate?
Find the net ionic equation of when nitric acid (HNO3) and solid potassium hydrogencarbonate react with each other:
What are metal oxides? List some examples.
Metal oxides: compounds containing a metal cation and an oxygen atom (O2-)
e.g.
What occurs when an acid reacts with a metal oxide?
Acid + metal oxide --> ionic salt + water
What are metal hydroxides? List some examples.
Metal hydroxides: ionic compounds formed from a metal cation and hydroxide ions (OH-)
e.g.
Sodium hydroxide NaOH
Barium hydroxide Ba(OH)2
Potassium hydroxide KOH
When added to aqueous solution, soluble metal hydroxides will ____ into its ___ and ____ ions.
dissociate , cation, hydroxide
What occurs when an acid reacts with a metal hydroxide?
What is an ionic salt made up of in an acid reaction?
Base’s cation + acid’s anion
The soluble ionic salt will ___ in water, breaking down into its ___ and ____.
Dissociate, anions, cations
Draw what occurs in this reaction:
Find the net ionic equation of when potassium hydroxide reacts with nitric acid:
What is a neutralisation reaction?
Reaction between an acid and base that forms a salt and water (and carbon dioxide when metal carbonate is a reactant)
What are antacids?
Antacids: weak bases that are used as medicines to treat symptoms caused by excess hydrochloric acid (HCl) in the stomach, such as ulcers, heartburn and indigestion, by neutralizing the acid.
Why can’t strong bases be used in the stomach?
Strong bases cannot be used as they will corrode the internal linings within the body + cause permanent damage
Examples of antacids:
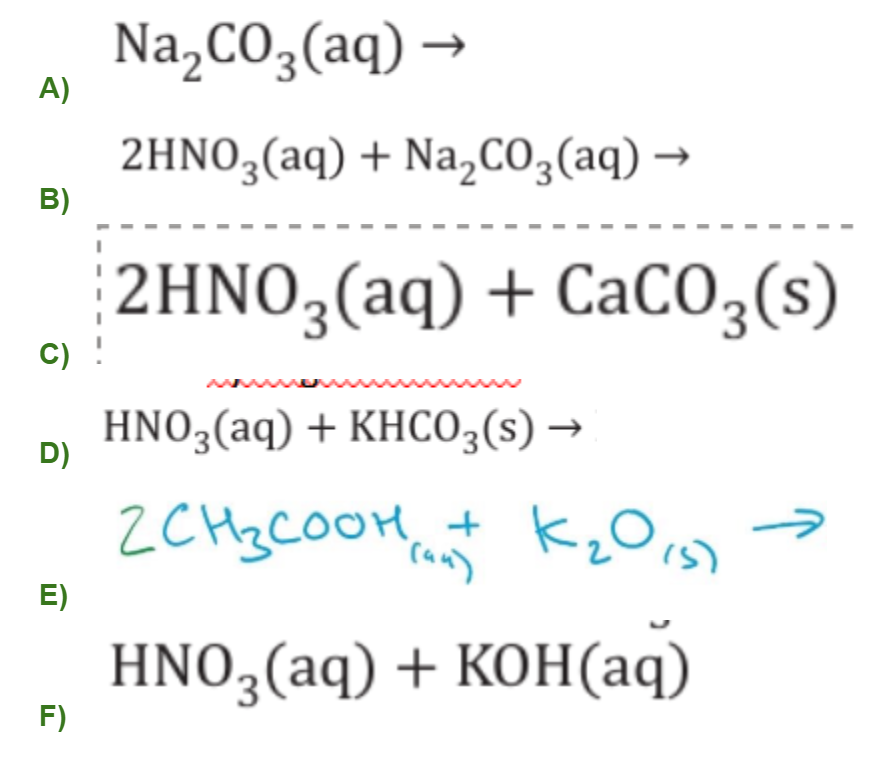
What type of acid reactions are these and find the products:
What is the autoionization of water/self-ionisation? State the chemical equation.
Refers to the process where water reacts with itself to form ions.
One molecule acts as the acid → donates the proton
The other molecule as the base → accepts the proton
Why does autoionization of water/self-ionisation occur to a very small extent?
Due to water being a relatively weak acid and base, where it only partially ionizes and dissociates in solution
Pure water at 25 ℃, the concentrations of both hydronium and hydroxide ions are ____.
1.00 × 10−7 M
What is the ionic product of water (Kw)?
Product of the concentrations of hydronium and hydroxide ions in water.
At 25 °C, the ionic product of water is always _____.
1.00 × 10−14 M2
In order to maintain a constant Kw, if the concentration of one ion increases, the other must ____.
decrease
Solve:
At 25 ℃, if there are 1.00 x 10-5 M of hydronium ions in pure water, how much hydroxide ions are there?
At 25 ℃, if there are 1.00 x 10-4 M of hydroxide ions in pure water, how much hydronium ions are there?
What is the baseline of a neutral solution?
It is the values of hydronium and hydroxide ions in pure water at 25 degrees celsius, where both ions have equal concentrations are equal to 1.00 × 10−14 M2