Intro to Biology - Midterm 1
1/69
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
70 Terms
Definition of Biology
The branch of science that deals with living organisms and their vital processes.
Characteristics of living things (7)
1. Made of similar chemical composition (biomolecules: CHON + PS; inorganic molecules)
2. Have a metabolism
3. Reproduction
4. Homeostasis/Regulation
5. Respond to stimuli
6. Growth and development
7. Adaptation and evolution
Steps of the Scientific Method (7)
1. Make an observation
2. Ask a question
3. Propose a hypothesis
4. Test hypothesis
5. Results
6. Draw conclusions
7. Communicate results
The Natural Sciences
The science of wanting to understand the world and the universe. 2 branches: Life sciences/biology (living parts of nature) & physical science (non-living parts of nature)
Applied Science vs Basic Science. Examples.
Applied science: to solve specific problems in world; ex: COVID vaccine
Basic science: to expand knowledge, knowledge for knowledge's sake (not for commercial or public use); ex: photosynthetic reactions
Levels of organization of living things (10)
Biosphere (living part of Earth/ecosystem)
Ecosystem (community & its environment)
Community (interacting groups of various pop. living together)
Population (group of organisms of 1 type in same area)
Organisms (individual living things)
Organs (collection of tissues that form a functional unit)
Tissues (groups of similar cells that come together)
Cells (smallest functional unit of life)
Organelles (components of eukaryotic cells)
Molecules (groups of atoms)
What is the source of diversity of life? How are organisms named and organized?
Evolution is the source of diversity of life. Living things are organized according to similar structure, physical and/or genetic characteristics, aka taxonomy.
Species
A group of organisms that can reproduce with each other in nature and produce fertile offspring
Genus
A group of similar species
Family
A group of similar genera (plural genus)
What is taxonomy? Taxonomic system of living things
The science of naming and classifying organisms into groups of increasing breadth.
What is a phylogenetic tree? & how to read it?
A diagram that represents evolutionary relationships among organisms based on similarities and differences in genetic and/or physical traits (hypotheses, not definitive facts). A branch point represents ancestors & show when an ancestor is believed to have diverged to form 2 new species. The length of branches is proportional to the amount of time that has passed.
Domains of life (3)
Bacteria, Archaea, Eukarya
Kingdoms of life (6)
Eubacteria/bacteria
Archaea
Protista
Fungi
Plantae
Animalia
Eubacteria/bacteria Kingdom characteristics
unicellular, prokaryotic, heterotrophs & autotrophs
Archaea Kingdom characteristics
unicellular, prokaryotic, live in extreme evironments
Protista Kingdom characteristics
unicellular, eukaryotic, no cell wall, heterotrophs & autotrophs, most are aquatic
Fungi Kingdom characteristics
most multicellular (except yeast), eukaryotes, heterotrophs, important for ecosystem of soil
Plant Kingdom characteristics
multicellular, eukaruyotic, photosynthetic autotrophs, VITAL for life on Earth
Animal Kingdom characteristics
multicellular, eukaryotes, heterotrophs, digestive systems & have to trap food & motile
What are viruses?
acellular, NOT made of cells, not alive, made of DNA or RNA w/ protein coat, obligate intracellular parasites that invade cells in order to survive & reproduce
Atoms and molecules in living things
Carbon + Hydrogen, Nitrogen and/or Oxygen
Organic vs inorganic biomolecules
Organic: made of mainly Carbon atoms forming large structures (carbs, proteins, nucleic acids, lipids)
Inorganic: do not contain C-H bonds (water, salts, gases…)
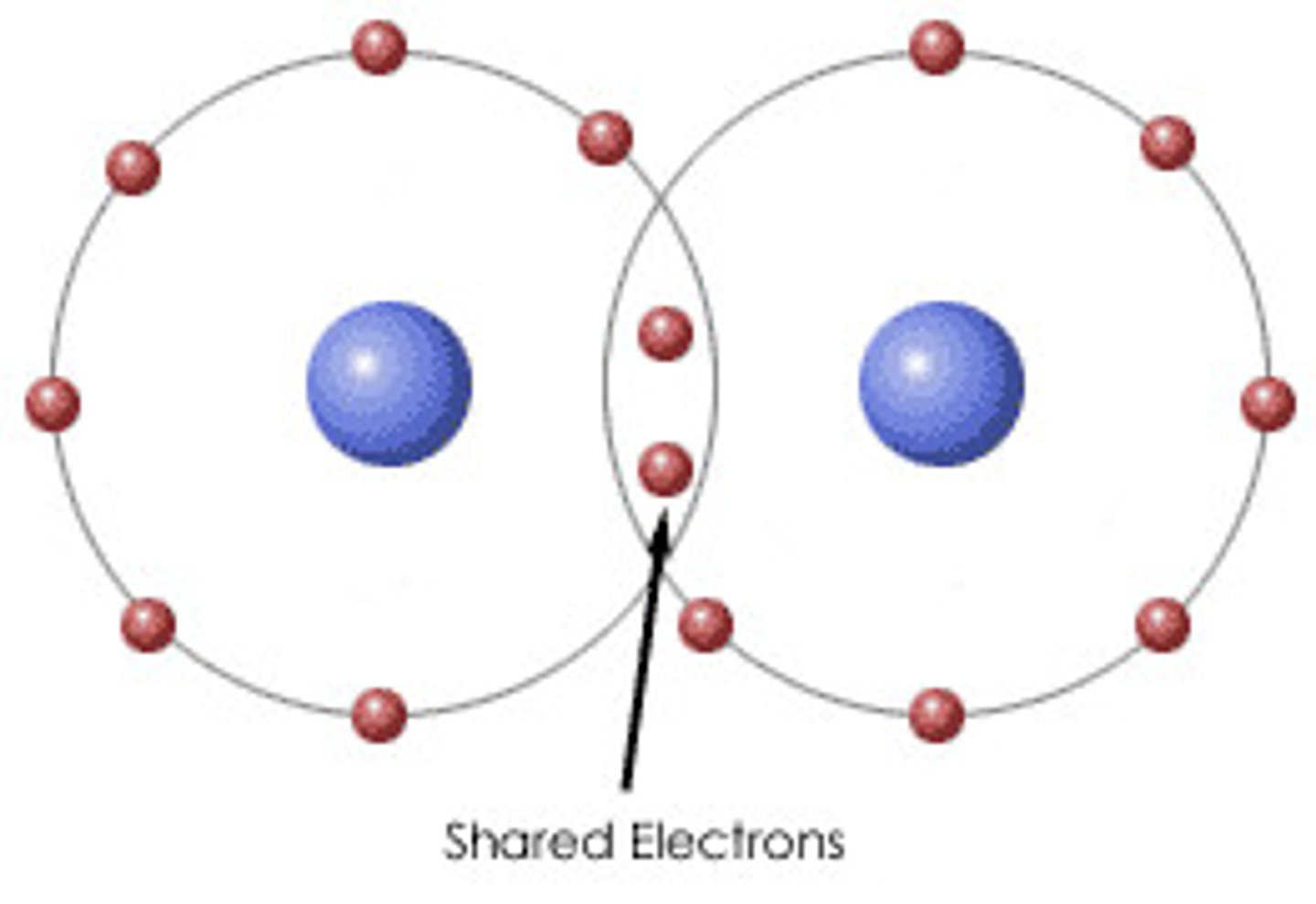
Importance of covalent bonds and characteristics
Form between elements to form biomolecules
Involve sharing electrons between 2 atoms, have definite orientations in space relative to one another -> many 3D orientations and shapes (ex: C-C bonds, 4 bonds of C)
Strongest type of bond, can be polar or nonpolar
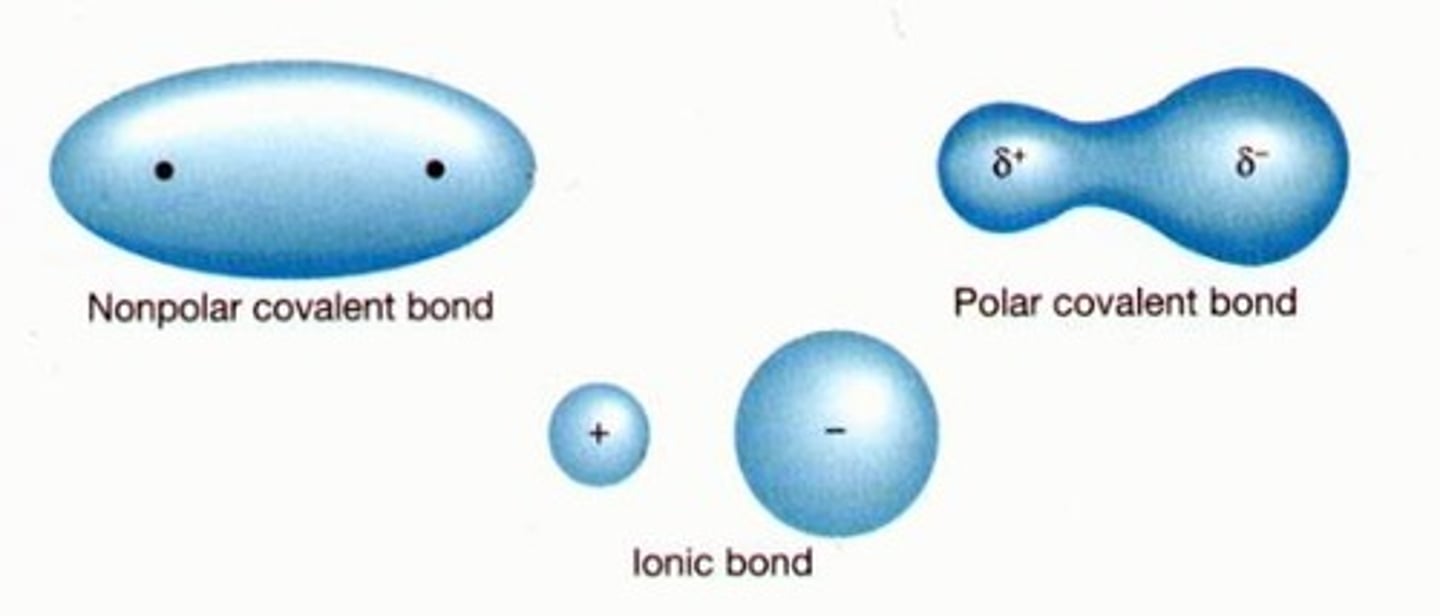
Polar vs. non-polar covalent bonds
Polar: when atoms are different & have different EN, and electrons are attracted unequally by atoms (δ+, δ-)
Non-polar: when atoms are the same of of similar EN, and electrons are shared equally
Hydrogen bonds. What are they? Importance in living organisms.
Chemical bonds involving electrostatic attraction btwn an H atom (δ+) and another atom (δ-), in a polar molecule
Determine the interaction of biomolecules.
Can be broken easily, except when many add up together to represent strong forces to acquire a molecular shape
Can be formed intramolecularly and extramolecularly
Shapes in biomolecules.
Carbs, proteins, nucleic acids, and lipids
Water in living things
Essential for life, the most abundant inorganic molecule in living organisms, H-bonding makes it very suitable for life
Properties of water making it suitable for supporting life (5)
Solvent of life
Special properties of cohesion & adhesion
High surface tension
High heat capacity
Expands upon freezing
Water as a solvent
Water is a good solvent due to its polarity
Polar H2O molecules surround other polar molecules, making H-bonds and surrounding other +/- ions
Hydrophilic and hydrophobic molecules. What causes them to be hydrophilic or hydrophobic?
Hydrophilic- polar molecules/charged ionic molecules, most cellular molecules, dissolve in water, have affinity for H2O, charged and polar molecules dissolve readily in H2O.
Hydrophobic- non-polar/uncharged molecules, oil molecules, don't dissolve in water, no H2O affinity, nonpolar or uncharged molecules don't dissolve in H2O
Monosaccharides: characteristics- how they are classified ("n" of Carbons, aldoses, ketoses). What are alpha and beta monosaccharide rings? Recognize the general structure.
aka simple sugars, one sugar molecule
# of Carbons: n=3 Trioses, n=4 Tetraoses, n=5 Pentoses, n=6 Hexoses
Aldoses (have aldehyde group) vs Ketoses (have ketone group)
Can exist in linear chain or ring-shaped structure
Ring-shaped structure can have 2 different hydroxyl group arrangements (OH) around anometric carbon (C#1): below-Alpha, above-Beta
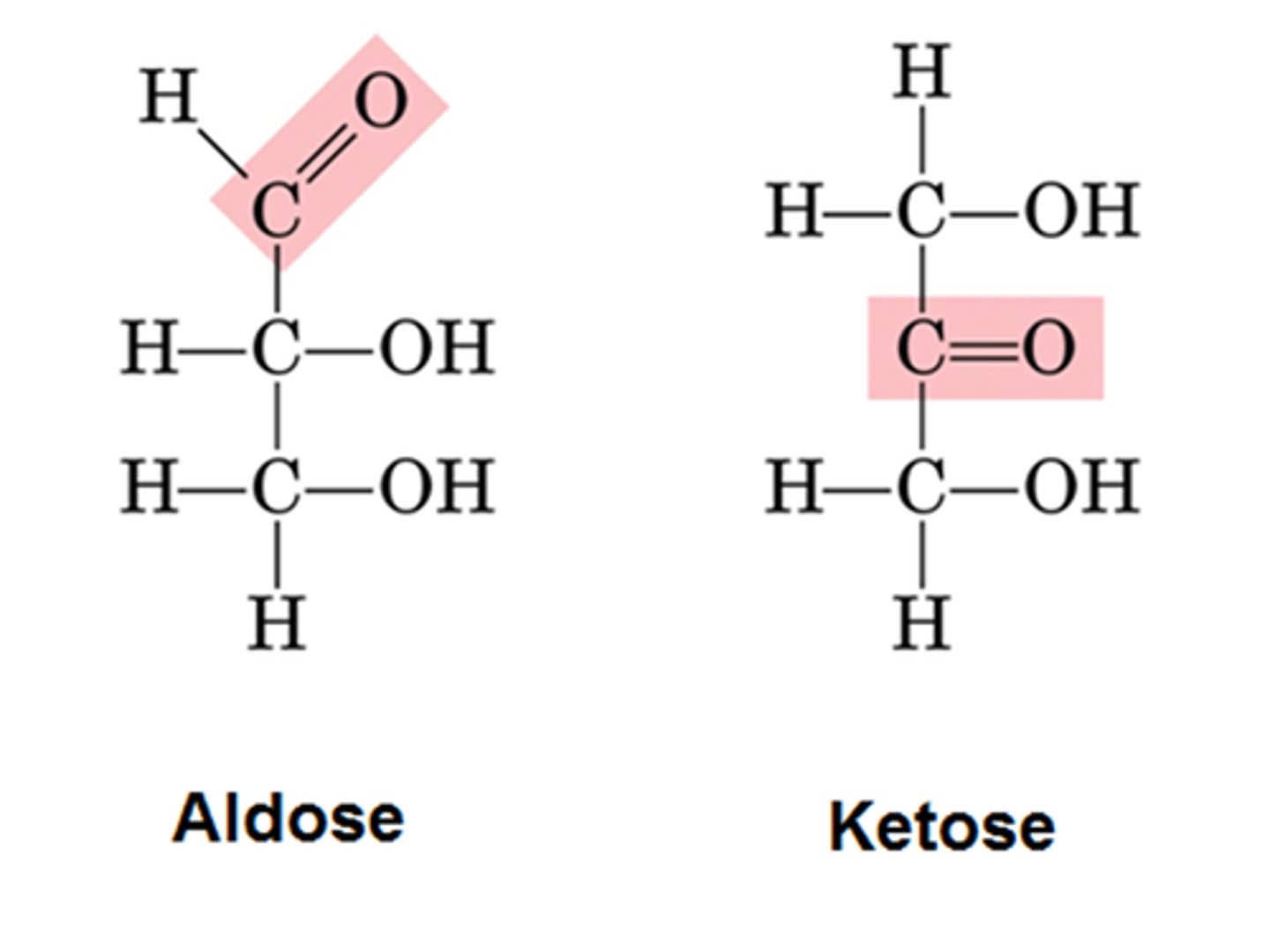
Classification of carbohydrates.
Biomolecules w/ a general formula (CH2O)n'
Most are hydrophilic
Classified in: Monosaccharides, Disaccharides, Oligosaccharides, Polysaccharides
Most common monosaccharides and their function
Pentoses: Ribose (RNA) and Deoxyribose (DNA)
Hexoses: Glucose (all cells), Galactose (milk), Fructose (fruit), (all provide energy to cells & used to build larger molecules)
Disaccharides- glycosidic bonds. Condensation (dehydration) vs hydrolysis.
Alpha (1-4)
Form when 4 monosaccharides join & are bound by a glycosidic bond (covalent bond
Condensation/dehydration reaction- H2O is released, "hydrolytic reaction"
Hydrolysis- a molecule is broken down into two fragments by the addition of H2O, which splits the bonds in the compound
Most important disaccharides and their functions. Need to know the three types and the monosaccharides that make them.
Sucrose, table sugar, one glucose and one fructose molecule, energy
Lactose, milk, one glucose and one galactose, energy
Maltose, malt sugar, two glucose molecules, energy
Oligosaccharides: what they are and their functions.
Several monosaccharides (3-10) bound together by glycosidic bonds, attached to proteins in membrane (glycoproteins) or phospholipids in membrane (glycolipids)
Cell to cell communication & recognition, hormone receptors, virus receptors, signaling btwn cells, red blood cell types (A, B, AB)
Polysaccharides
Made of long chains of monosaccharides bound together by 100s of glycosidic bonds
Storage polysaccharides: Starch - components/structure (amylose & amylopectin, properties, alpha bonds. Where do you find starch? Importance of starch. Iodine & starch
Long term energy storage for plants, excess glucose (leaves, stems, roots, seeds), provides food for plant embryos
Food source for animals
Amylose: 20%, linear polysaccharide composed of glucose units joined by the a-1,4-glycosidic linkage, coils like a ring, stabilizes by forming H-bonds (when coiled, has room for iodine molecule)
Amylopectin: 80%, branched-chain polysaccharide made of glucose units linked by a-1,4-glycosidic bonds, branched at a-1, 6 glycosidic bonds
Digestion of starch
Starch digestion: digestive enzymes break down starch in our bodies & glucose molecules are absorbed, have a-glycosidic bonds allowing flexibility + ease of digestion
Glyocogen- components/structure, function, which cells? When glycogen is made? When is it hydrolyzed?
Excess glucose in blood -> stored in form of glycogen in granules in liver & muscle cells
Blood sugar level drop -> breaking of glycogen to free glucoses
Structurally similar to amylopectin glucose, a(1-4) branched by a(1-6)
Highly branched & shorter branches
When fasting, animals can draw on these glycogen reserves (break bonds to release glucose to blood)
Structural polysaccharides: Cellulose - components, structure, properties, beta bonds. Where do you find it? Which organisms can digest it? Importance of cellulose in nature.
Main components in tough plant cell walls
Most abundant of all carbs, over 50% of all carbon in vegetables
ex: filter paper, cotton fibrils, wood, leaves
Bonds cannot be broken by humans: "insoluble fiber"
Some microbes, fungi, insects (ex: termites) can digest it
Many herbivores have symbiotic relationships w/ microbes that can digests it, allowing them to digest cellulose and use it for energy (ex: cows, giraffes, zebras)
Structural polysaccharides: Chitin- properties. Structure. Beta bonds. Which organisms? Function.
Gives strength to exoskeletons of crustaceans, insects, cell walls of fungi
Structurally similar to cellulose but built from N-acetyl-glucosamine subunits thru B bonds
Not soluble in water
LIPIDS: definition, characteristics
Triglycerides, Phospholipids, Steroids
Mostly hydrophobic bc consist of mostly hydrocarbon (C-H), nonpolar
Fats separate from water bc water molecules hydrogen-bond to each other and exclude facts
Only class of large biological molecules that DO NOT include true polymers (no long chains)
Fats / Triglycerides: Structure. Fatty acids (types- saturated & unsaturated). Liquid and solid fats. Where do you find them? Functions
Made of 1 glycerol (3 carbon molecules w/ a hydroxyl OH group attached to each carbon) & 3 fatty acids (long hydrocarbon chains C-H w/ a COOH at one end)
Fatty acids (FA): vary in length (# of C) & in # of locations of double bonds
FA- Saturated (all single bonds, packs at room temp., solid, ex: lard, butter, coconut) vs unsaturated (>1 double bonds, does not pack liquid, ex: oils)
Fats: Saturated vs. unsaturated
Energy storage in plants & animals
Plants accumulate fats in seeds
Humans & mammals store long-term food reserves in adipose cells (adipose tissue cushions vital organs & insulates body)
Saturated (made from saturated fatty acids, solid at room temp., most animal fats, can lead to heart disease, ex: beef, lamb, dairy, mayo) vs unsaturated (made from unsaturated fatty acids, liquid at room temp., most plants & fish fats, can be monounsaturated (1=) or polyunsaturated (>/= 2=), can lower bad cholesteral, ex: canola oil, avocado, seeds, nuts)
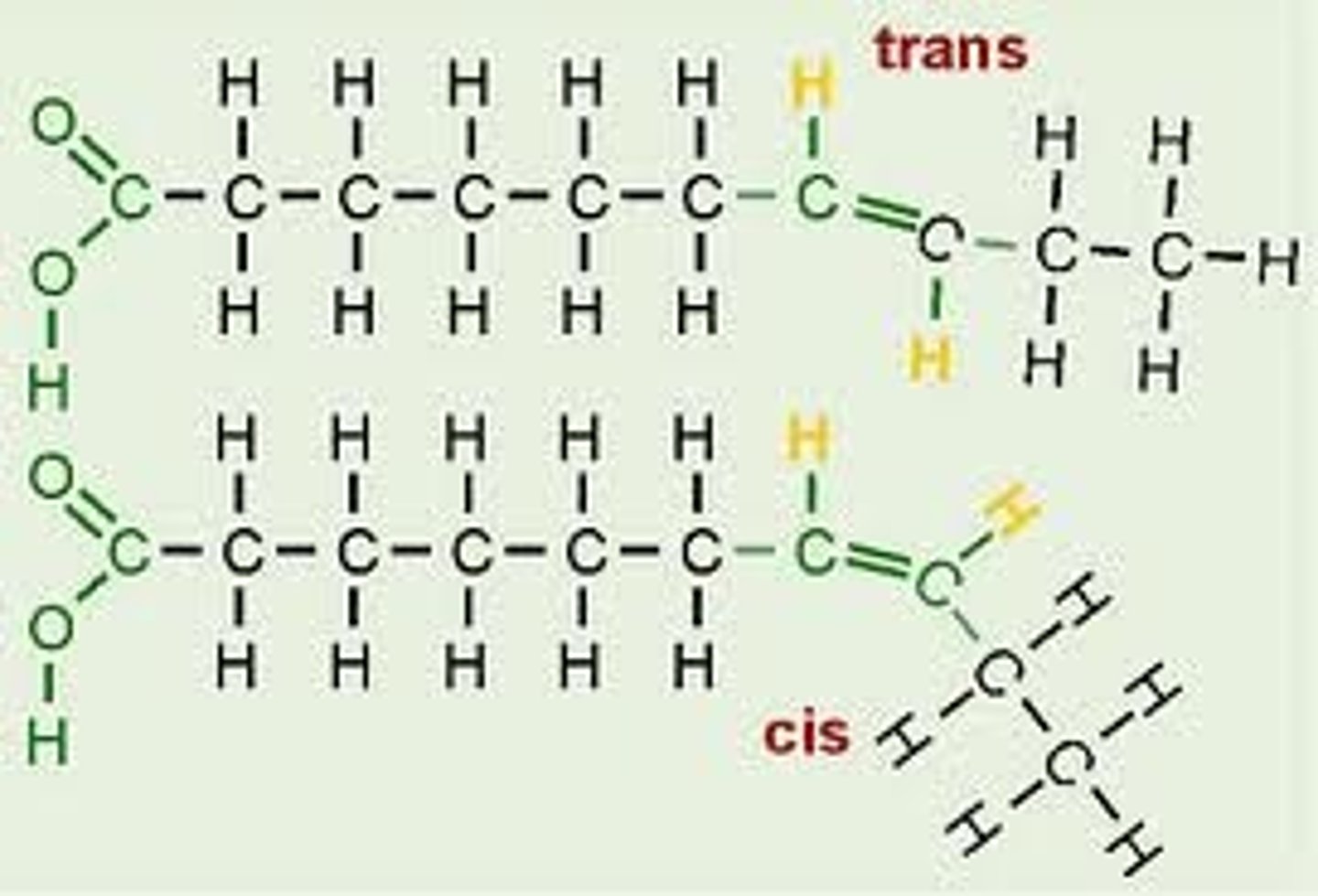
Cis fats vs. trans fats- what is the "problem" with the trans fats? Do they appear in Nature. Health issues.
Cis fats: naturally occurring unsaturated fats, H atoms are on same side of double bond
Trans fats: made by man-made/industrial hydrogenation of oils; conversion of cis fats to a cheap fat w/ longer shelf life by adding H to oils, not naturally occurring, H atoms on opposite sides of double bond, increases risk of heart disease- very unhealthy
Phospholipids: Structure. Amphipathic molecule. What is their Function?
Made of: 1 glycerol, 2 fatty acids, phosphate group, R group
Tail: 2 fatty acid tails are nonpolar hydrophobic
Head: Phosphate-containing & alcohol (R) is polar hydrophilic
Both hydrophilic & hydrophobic in same molecule (called amphipathic), forms in bilayers in aqueous environment
Function: main component of cell membranes
Steroids: General structure. Functions.
Lipid characterized by a carbon skeleton consisting of 4 fused rings
Don't have fatty acids
Mostly hydrophobic
ex: cholesterol, vitamin D, sex hormones, bile salts, cortisone, anabolic steroids
Ex. of a steroid: Cholesterol- where in the cell? what is its function?
Amphipathic, component in animal cell membranes, precursor from which other steroids are made
Not in prokaryotes & plant cells, constitutes almost 25% of membrane lipids in animal cells
PROTEINS: What are they? Some functions. What determines the function of a protein?
Constitute most of a cell's dry mass
Execute nearly all functions in living cells
Functions arise from the huge # of diff. shapes they adopt, how they interact w/ other proteins, & variety of functions they can perform
ex: Enzymatic (acceleration of chemical reactions), defensive (protection against disease), storage (storage of amino acids), transport (transport of substances), hormonal, receptor, contractile & motor, structural, etc.
Structure: large polymers made of amino acids, made from different combos of 20 amino acids liked by peptide bonds (covalent)
Amino acids- general structure. Types of amino acids (the four groups) but you don't need to know the individual amino acids.
Contain 3 groups attached to central C: amino group, carboxyl group, side chain (R group) varies btwn 20 diff. amino acids
Differ in chemical properties due to differing side chains (R groups)
20 diff. types of amino acids w/ diff. side chains, which can be: hydrophilic (charged) / hydrophobic (nonpolar)
Linked by peptide bonds, forming a polymer (<50, peptide; >50, protein)
Peptide bond/ Polypeptides. What is a peptide and protein?
Each has a unique linear sequence of amino acids, w/ a carboxyl end (C-terminus) & an amino end (N-terminus)
Carboxyl group of 1 AA is linked to amino group of a 2nd AA
Peptide bonds made in cell by enzymes in ribosomes, can be broken by enzymes
One end of PP: free amino group
Other end of PP: free carboxyl group
N-ter -> C-ter direction
<50 AA: peptide
>50 AA: protein
Primary structure of a protein- What is it? What types of bonds hold amino acids together?
Sequence of AA determined by inherited genetic info (in genes, DNA)
3-letter, or 1-letter
Secondary structure of a protein- What is it? types. What types of bonds hold the secondary structure together?
Coils & folds that result from H-bonds btwn PP backbone
Typically a cell called a(alpha) helix & a folded structure called a B(beta) pleated sheet
Tertiary structure- What is it? what types of bonds hold the tertiary structure together?
Overall 3D shape of PP
Results from interactions btwn R groups of AA that make up protein, rather than interactions btwn backbone constituents
R group interactions include weak bonds, ex: hydrogen bonds, iconic bonds, hydrophobic interactions
Strong covalent bonds called disulfide bridges may reinforce protein's structure
Quaternary structure of a protein- What is it? what types of bonds hold a quaternary structure?
Exists only when protein is made of several subunits
Results when 2 or more PP chains (subunits) combine & work together to form 1 larger protein
ex: collagen- 3 PP coiled like a rope, hemoglobin- 4 PP, 2a & 2B chains
Protein denaturation: What does it mean that a protein denatures? How and why do proteins denature? Explain what happens when a protein is denatured. Which factors can denature a protein?
Loss of a protein's native structure
Biologically inactive (loses function)
Caused by anything that disrupts weak bonds that hold secondary, tertiary, & quaternary structure, so that protein loses its shape (protein unravels)
Factors: high temp., alterations in pH, high concentration of salts
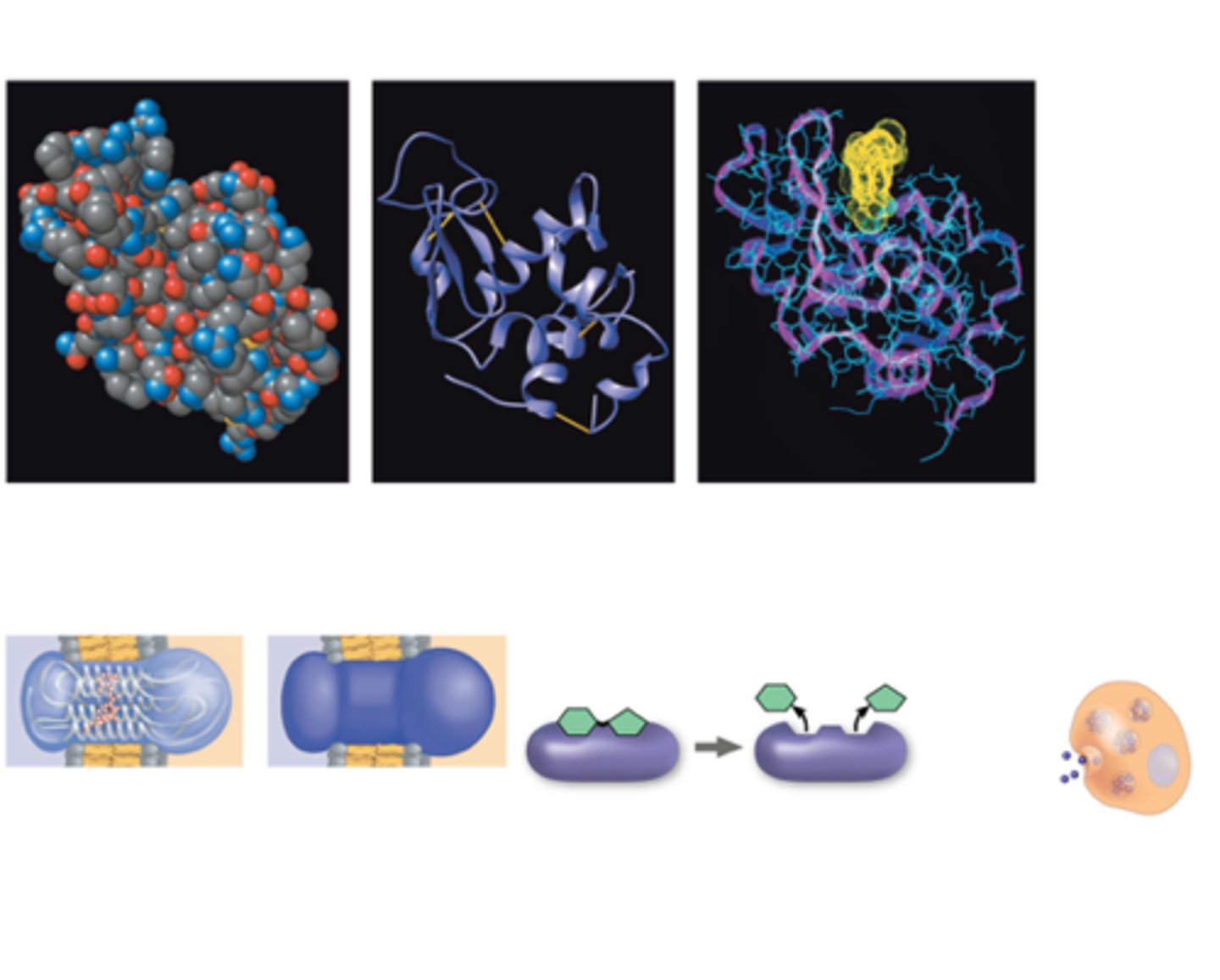
How are proteins depicted?
Globular & fibrous; can form filaments, sheets, rings, spheres
Online models: Backbone, ribbon, wire model, space-filling
MICROSCOPES: Magnification, Resolution, and Contrast- what are they and why are they important?
Magnification: how big we can make/amplify object
Resolution: how clear/sharp images are, provides clarity to magnified image, w/ more details (low-res vs. high-res)
^provided in microscope
Contrast: differences in intensity &/or color creates contrast, allowing features of speciman to become visible, ex: staining speciman, adding color, etc.
^req. external manipulation/ found in cells
Light microscope: Types of light microscope.
Visible light is passed through a specimen & then thru glass lenses
Can observe live or dead specimens can stain specimens to increase contrast
Types: Brightfield- poor contrast of unstained (only used on dead cells); Phase- good contrast, shows organelle contrast w/o staining needed (live cells); Fluorescent- fluorescent dye tags specific molecules inside cells
Electron microscope: Types of electron microscopes (SEM, TEM)
Black n white
Types: Scanning electron microscopes (SEMs)- focus a beam onto surface of a specimen (providing 3D images), used to study shape of cells; Transmission electron microscope (TEMs)- focus a bam of electrons thru a specimen, used to study internal structure of cells
Which microscope gives the highest resolution and magnification?
TEM
PROKARYOTIC CELLS: When did they appear on Earth?
Oldest, structurally simplest, most abundant
Dominated Earch for over a billion yrs before eukaryotes appeared
90% are unknown/undescribed
Bacteria & Archaea
Bacteria: General characteristics.
Small (0.005mm) in length
Unicellular
Lack a membrane-bound nucleus + organelles
DNA in region called nucleoid
Cytoplasm bound by plasma membrane
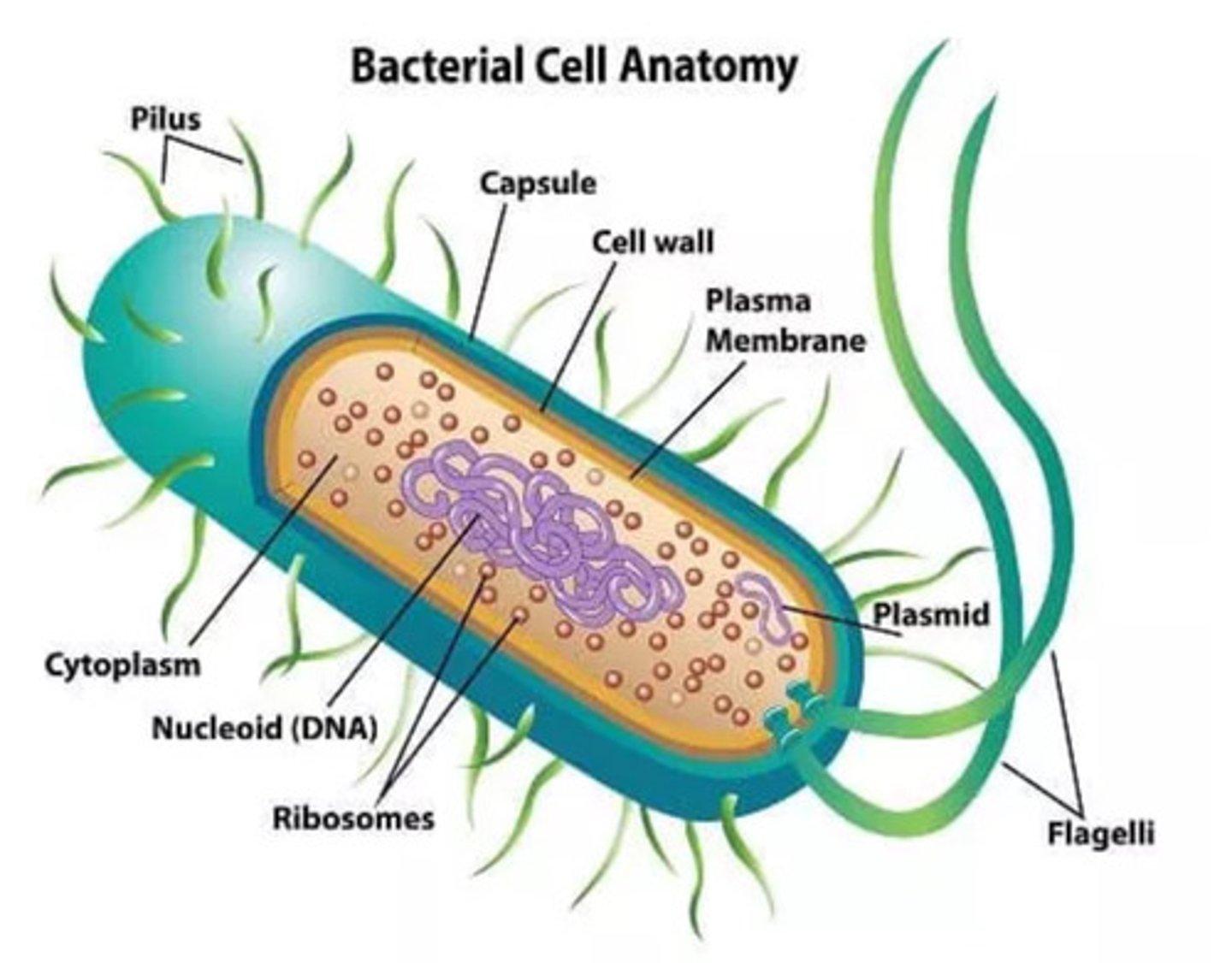
Parts of a bacteria cell, composition, what is gram stain?
Nucleoid
Plasmid
Cell wall
Flagella
Capsule
Fimbriae & Pili
Ribosomes
Slime & capsules
Can be stained w/ a gram stain: gram positive- bacteria have a thicker peptoglycan wall, purple; gram negative- bacteria contain less peptoglycan & don't retain dye, pink
Shapes of bacteria.
Coccus- spherical
Bacillus- rod-shaped
Spirillum- helical-shaped
Good bacteria: examples
Normal flora of intestines, bacteria involved in nutrient cycles, symbiotic bacteria (ruminents), bacteria used in research & genetic engineering, biomediation
Archaeas: examples, where do they live?
Extremophiles, live in extreme environments (ex: deep sea, hot springs, inside digestive tracts of some animals, anoxic muds of marshed, petroleum deposits underground, etc.)
Differences between Bacteria and Archaea
1. Have different cellular composition (plasma membrane and cell wall, esp.). No peptidoglycan in Archaea but peptidoglycan is present in Bacteria
2. They have different niches in which to grow. Archaea like extreme environments
3. Archaea is more ancient
4. Archaea is more similar to eukaryotic cells in its DNA replication, transcription/translation, & have histones