10.3 - Molecular Polarity
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
A molecule is polar if
difference in electronegativity is not the same between all bond
if the same between all bonds, ensure that molecular is shaped in a way that makes it cancel out
a molecule is nonpolar if
all the bonds have the same difference in electronegativity and molecule is shaped in a way that it cancels out
are diatomic molecules polsar or nonpolar
nonpolar
what is a cisisomer
when the same atom(s) in a compound are adjacent in lewis structure
what is a trans isomer
when the same atom(s) is a compound are opposite of eachother in lewis structure
memorize electronegativyt on formula sheet
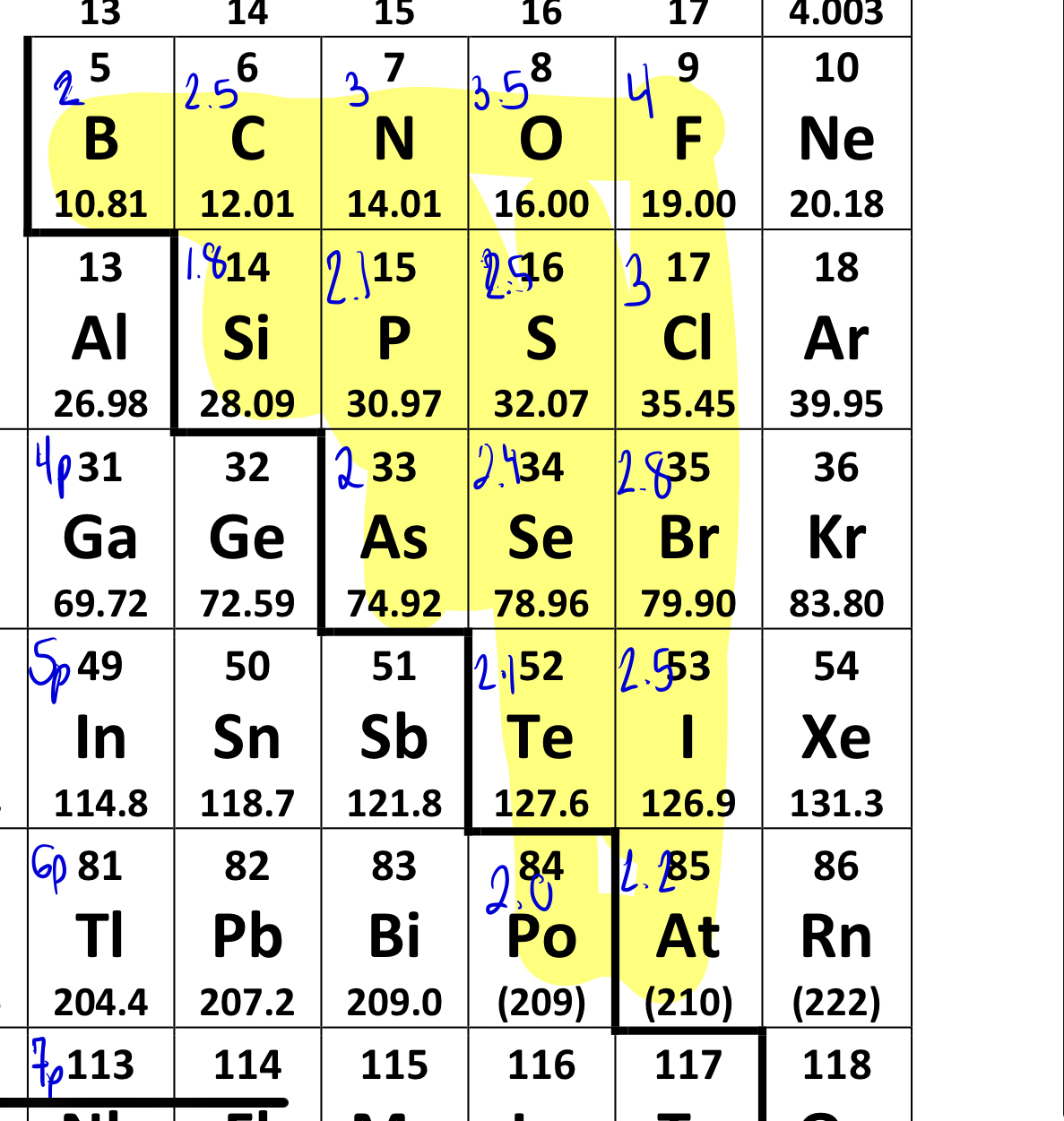
are complete (MEANING NO LONE PAIRS) linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral moleulcar shapes polar or on polar?
nonpolar, if there are lone pairs they are polar