Chemistry - Common Midterm Study Guide
1/80
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
81 Terms
Which scientist claimed, “All elements are made up of atoms. It is impossible to divide or destroy an atom”
John Dalton
Which scientist claimed, “All atoms of the same element are identical”
John Dalton
Which scientist claimed, “Atoms of different elements are different”
John Dalton
Which scientist claimed, “Atoms of different elements combine to form a compound. These atoms have to be in definite whole number ratios”
John Dalton
Which scientist claimed, “Chemical reactions occur when atoms are separated, joined, or rearranged. Atoms of one element are never changed into atoms of another element as a result of a chemical reaction”
John Dalton
What was the theory that stated all matter is composed of extremely small particles called atoms and atoms of the same element were identical in size, mass, and other properties?
Dalton’s Atomic Theory
Which scientist worked on a cathode ray tube experiment?
JJ Thompson
What is a cathode ray tube?
A glass tube containing two electrodes with a high voltage applied
Describe JJ Thompson’s cathode ray experiment.
A cathode ray was shot between two charged plates (one positive and one negative) toward a fluorescent screen. The angle at which the ray bent was analyzed.
What are cathodes made of?
Metals
What was the purpose of JJ Thompson’s cathode ray experiment?
To learn if the particles emitted had a charge
What happened when Thompson’s cathode ray was turned on?
A particle beam passes through a hole in the anode, hitting a glass wall on the opposite end of the tube.
What happened when charged plates were added to JJ Thompson’s cathode ray experiment?
The ray bent away from the negative plate and toward the positive plate. This showed the ray was negative (composed of electrons). The same thing happened when magnets replaced the plates.
What did Thompson find from his cathode ray experiment?
The mass and charge of the particles in the stream (ray).
Who preformed the gold foil experiment?
Ernest Rutherford
How was the gold foil experiment set up?
Alpha particles were shot at a thin sheet of gold foil surrounded by a detection screen. Most of the particles shot passed directly through the gold foil. Some, however, were deflected (slightly and directly back), showing there was a positive center with mass.
What were the results of the gold foil experiment?
Particles went straight through
Particles were slightly bent
Particles deflected back without passing through the foil
What did Rutherford conclude from his gold foil experiment?
Most of the matter in an atom is grouped in a single location (nucleus) & that the matter in this location is positively charged and that most space inside an atom is empty
What part of John Dalton’s atomic theory was disproved by the cathode ray tube experiment?
The discovery of the electron; atoms were divisible
What conclusion did Rutherford make regarding the atom for each of the 3 main observations?
There is a positively charged center in the atom called a nucleus
Nearly all of the mass in the atom reside in the nucleus
Electrons revolve around the nucleus in circular paths
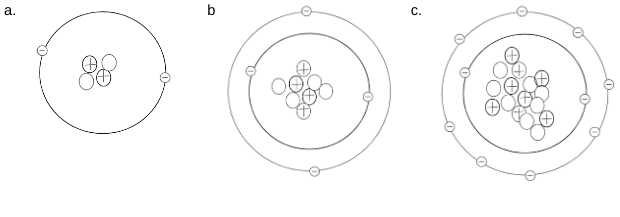
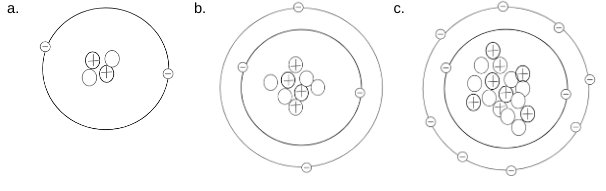
Which of the 2 following atomic models would be considered stable? Why?
a & c; There outer valence shells are full. A has a full shell of 2 (Helium) and C has a complete octet (8 electrons) and is full.
In order to create a neutrally charged nitrogen atom with an atomic mass of 15 amu how many of each subatomic particle would you need?
Protons: 7; Neutrons: 8; Electrons: 7
If the image below was the nucleus of an atom, how many electrons would you need in order for the atom to be neutral?
6 Electrons
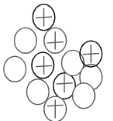
Name the elements in each drawing below
a.) Helium b.) Beryllium c.) Oxygen
A student records the volume of a gold nugget to be 6.75 cm3.
What other information would the student need in order to calculate the density of the gold? (*Hint: what is the formula for calculating density?)
Density is equal to mass divided by volume (d=M/V). You would need the mass of the gold nugget as well.
A student records the volume of a gold nugget to be 6.75 cm3. The mass of the gold nugget was 130.275 g. What is the density of the gold nugget? (Make sure to report answers with correct significant figures and units.)
d=m/v → 130.257 g / 6.75 cm3 = 19.2973 g/cm3 (6 sig figs)
A student records the volume of a gold nugget to be 6.75 cm3.
What would be the density of a gold nugget that was only 50.0 g?
d=m/v →50.0 g/6.75 cm3= 7.41 g/cm3 (3 sig figs)
A student used a calorimeter to compare the heat capacity of copper and water. First, the student found the mass of the copper cylinder and recorded it to be 40.125 g. The mass of the water that was placed in the calorimeter was recorded as 49.081 g. The copper cylinder was then heated to 100.0 °C and placed inside the calorimeter with the water sample at a starting temperature of 23.2 °C. The calorimeter is left until the temperature inside is uniform and stable. The final temperature of the contents in the calorimeter (water and copper sample) is recorded as 28.8 °C.
Which statement BEST compares the heat capacities of copper and water?
The heat capacity of copper is greater than the heat capacity of water because the water experiences a much smaller change in temperature.
The heat capacity of water is greater than the heat capacity of copper because the copper experiences a much smaller change in temperature.
The heat capacity of copper is greater than the heat capacity of water because the copper experiences a much smaller change in temperature.
The heat capacity of water is greater than the heat capacity of copper because the water experiences a much smaller change in temperature.
4
A student used a calorimeter to compare the heat capacity of copper and water. First, the student found the mass of the copper cylinder and recorded it to be 40.125 g. The mass of the water that was placed in the calorimeter was recorded as 49.081 g. The copper cylinder was then heated to 100.0 °C and placed inside the calorimeter with the water sample at a starting temperature of 23.2 °C. The calorimeter is left until the temperature inside is uniform and stable. The final temperature of the contents in the calorimeter (water and copper sample) is recorded as 28.8 °C.
Which statement describes how heat energy is transferred in the experiment?
Heat energy is transferred from the copper to the water which causes the temperature of the copper to decrease and the temperature of the water to increase.
Heat energy is transferred from the water to the copper, which causes the temperature of the copper to decrease and the temperature of the water to increase.
Heat energy is transferred from the water to the copper, which causes the temperature of the copper to increase and the temperature of the water to increase.
Heat energy is transferred from the copper to the water, which causes the temperature of the copper to increase and the temperature of the water to increase.
1
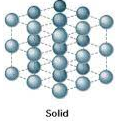
Describe how atoms are arranged in a solid
Closely packed together; Definite Shape; Crystallize structure
Describe how atoms are arranged in a liquid
Atoms roll past one another; Particles are slightly further apart compared to metals

Describe how atoms are arranged in a gas
Widely spaced out; Randomly arranged; Can be compressed or squashed

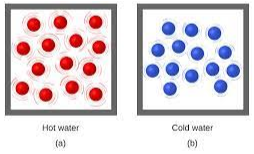
Which water molecules have more kinetic energy? Explain your reasoning.
Hot Water (A) because the molecules contain more energy meaning they have faster movement.
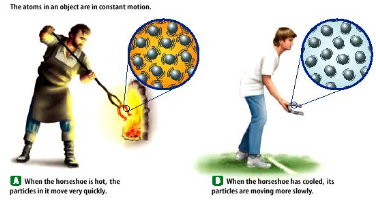
While the metal atoms in the horseshoe are in a solid phase at both of these temperatures, they are moving at different rates. Which horseshoe has atoms that are moving slower? How can you tell?
The atoms in the hot horseshoe. You can tell based off the temperature of the atom (the hotter the atom is, the more energy it carries and vice versa)
Electrons and protons (attract/repel) each other.
Attract
As an electron gets closer to the nucleus the (attraction/repulsion) to the nucleus gets (stronger/weaker).
Attraction; stronger
For an electron to move from an energy level close to the nucleus to an energy level far from the nucleus it would need to (absorb/emit) energy.
absorb
When an electron returns from an energy level far from the nucleus to an energy level close to the nucleus it would (absorb/emit) energy.
Emit
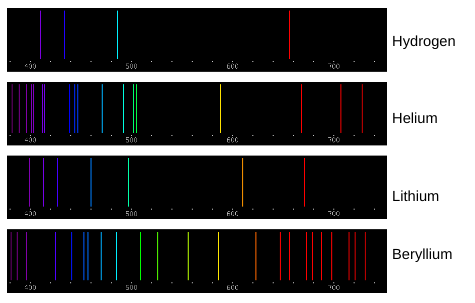
Which color of light corresponds to the shortest wavelength?
Violet
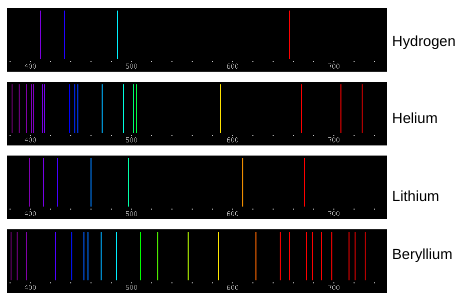
Which color of light corresponds to the longest wavelength?
Red
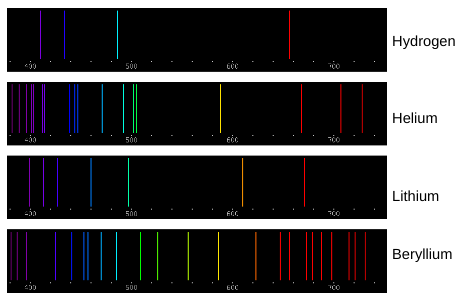
Which color of light has the most energy?
Violet
Which color of light has the least energy?
Red
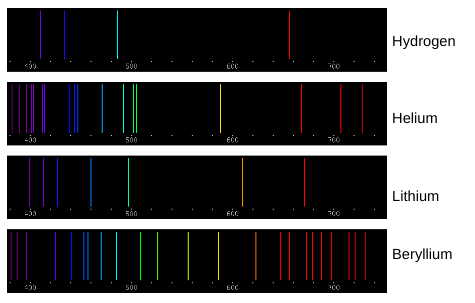
“The emission spectra lines for atoms of different elements are like fingerprints for humans.” How do the spectral lines shown above support this statement?
Each element has unique spectra lines. An element can be determined by burning it and seeing what spectra lines are emitted. If certain spectra are emitted and others are not, it will only correspond to one specific element. Burn Test
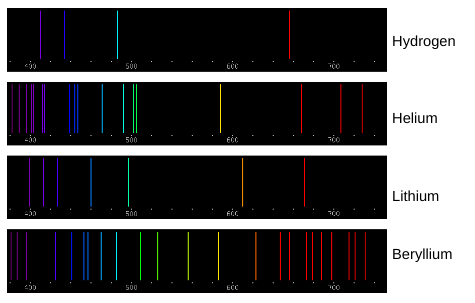
Elements can be identified by their individual spectral lines. Why does each element have its own unique set of emission spectra lines?
Each element has its own unique set of emission spectra lines because each element requires a unique amount of energy to move their electrons up and down an energy shell. Atoms absorb energy when their electrons move up an energy shell and that same energy is released when the electron moves back down. Each color of light has a unique energy level so each atom has unique colors when they release energy.
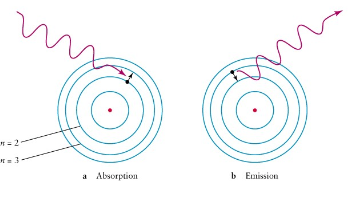
What is it called when electrons enter an excited state and move up energy levels (n=2 to n=3)?
Absorption
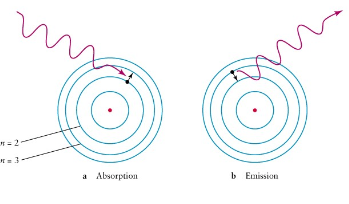
What is it called when electrons within an atom release energy and return to the grounded state (n=3 to n=2)?
Emission
When does ionizing radiation occur?
When electromagnetic radiation has enough energy to remove the electron from an atom, causing it to form an ion.
Rate the following electromagnetic radiation from most likely to be ionization radiation (highest energy) to least likely (lowest energy): radio waves gamma rays, visible light, x-rays, ultraviolet, microwaves, infrared
Gamma, x-ray, ultraviolet, visible, infrared, micro, radio
High energy is directly proportional to what other attribute of electromagnetic radiation?
Wavelength
Flame Test
One way in which unique emission spectra can be used to identify elements. An unknown metal is heated by a flame and the color of the flame is observed.
Strontium flame color:
Red magenta
Sodium flame color:
Yellow
Potassium flame color:
Violet
Calcium flame color:
Red orange
Copper flame color:
Green
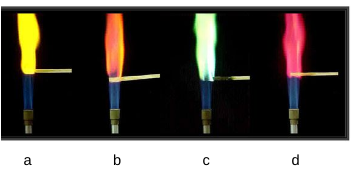
Identify which metal from the table is being heated in the flame test image above:
a. Sodium b. Calcium c. Copper d. Strontium
EXPLAIN why the metals must be heated before the colored light is emitted.
The electrons in the element must be “excited” first to make them jump up an energy level. Once they are at a higher energy level, the can drop back down to ground state, releasing light.
EXPLAIN why the different elements produce different colors in the flame test.
Different elements require different amounts of energy to excite their electrons. This means that as electrons from different atoms drop back to ground state, different amounts of energy is released, resulting in different colors.
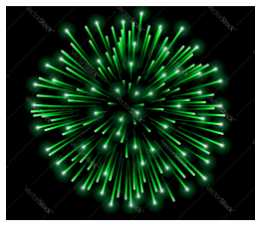
In the picture of a green firework, which compound was MOST LIKELY used when creating the firework?
Calcium sulfate
Sodium chloride
Copper (II) chloride
Strontium nitrate
Copper (II) chloride
What are intermolecular forces? Give an example
Attractive and repulsive forces between molecules of a substance.
Ex: Water molecules being attracted to one another (cohesion)
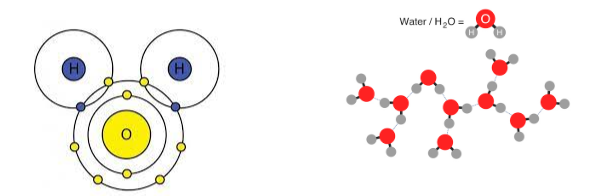
What are intramolecular forces? Give an example
Forces that hold atoms together within a molecule.
Ex: Ionic bonds, dipole-dipole, Van Der Waals, etc.
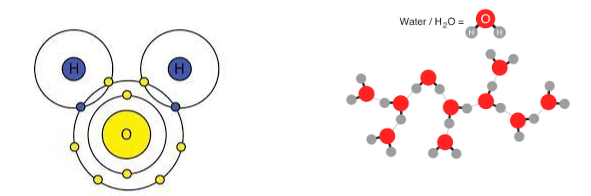
Accurately describe the distribution of charges in a water molecule
-
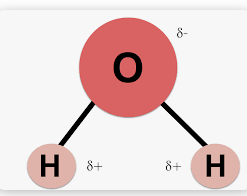
What is a hydrogen bond?
Any bond that involves a hydrogen atom being attached to another atom. Examples include oxygen, nitrogen, fluorine, or other polar covalent bonds.
What are metallic bonds?
A bond exclusively between metals. Creates a bulk of metal atoms which are conductive and malleable.
Ionic compounds form between (metals/nonmetals) and (metals/nonmetals).
Metal; nonmetal
Covalent compounds form between (metals/nonmetals) and (metals/nonmetals)
Nonmetal; nonmetal
Place the 9 compounds in the chart below to classify them according to their bond type.
CCl4 -Ionic
NaCl-Covalent
CO-Covalent
MgCl2 -Ionic
K2O -Ionic
FeO-Ionic
NO-Covalent
NH3-Covalent
PCl5-Covalent
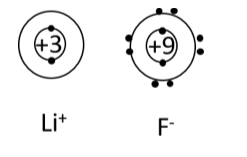
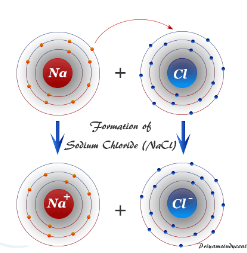
Ionic Bond
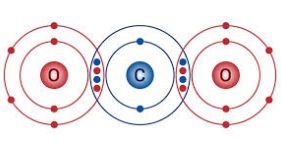
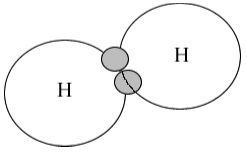
Covalent Bonding
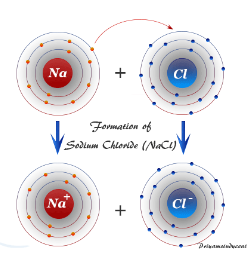
Consider the diagram of sodium chloride above. Describe what is happening in order for the ionic bond to form.
Sodium (metal) is giving its extra electron to chlorine. This gives sodium a full outer octet and it gives chlorine a full outer octet.
Describe how ionic bonds are formed. Make sure to use the key terms: electronegativity and valence electrons in your description.
Ionic bonds form between metals and nonmetals. Nonmetals have higher electromagnetivity than metals, meaning that the nonmetals want an electron more than the metals do. This causes the metal to “lose/give” its electrons to the nonmetal until both are stable (have an octet).
Most atoms achieve stability through gaining or losing electrons so that the valence shell is a full octet (8 electrons). What is the exception to this rule?
a. the d sublevel expands the valence shell to 18 electrons
b. some elements are more stable with 7 electrons than a full octet
c. the first energy level can only fit 2 electrons, making a full valence shell a duet
d. some elements are only stable with 9 electrons
C
The Periodic Table of Elements is organized based on multiple characteristics. Which is the first characteristic the table is organized by?
a. reactivity
b. similar chemical properties
c. average atomic mass
d. proton number
D
Which elements are the LEAST reactive? (aka: most stable)
a. atoms with a full valence shell
b. atoms 1 electron away from a full valence shell
c. atoms with a half-filled valence shell
d. atoms with a half-filled p sublevel
A
Which elements are the MOST reactive? (aka: least stable)
a. atoms with a full valence shell
b. atoms 1 electron away from a full valence shell
c. atoms with a half-filled valence shell
d. atoms with a half-filled p sublevel
B
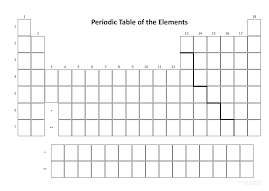
5. Draw arrows to show INCREASING atomic radius for both the group (column) and period (row) trends:
Increase down a group & decrease across a period
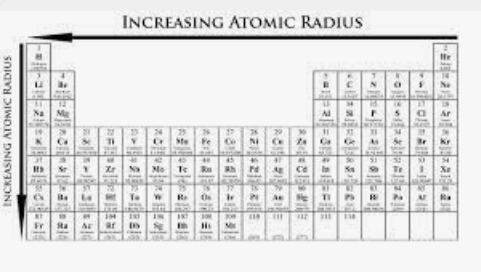
Order the following elements from smallest to largest atomic radius: Sr, Ra, Ba, Be, Mg
Be, Mg, Sr, Ba, Ra
Order the following elements from smallest to largest atomic radius: Ar, Na, Al, S, Cl
Ar, Cl, S, Al, Na
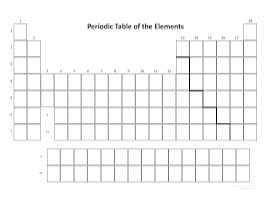
Draw arrows to show INCREASING electronegativity for both the group (column) and period (row) trends:
Decrease down a group & increase across a period
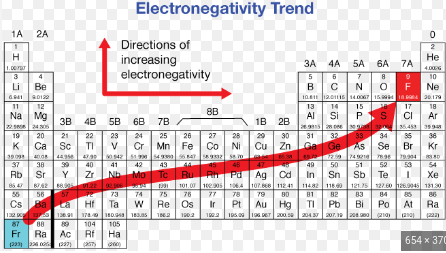
What element is the MOST electronegative of the following?
chlorine
sulfur
iodined
carbon
Chlorine
Which element is the LEAST electronegative of the following?
fluorine
lithium
francium
helium
Francium