Chem 101 UNC Chapel Hill | Exam 2 | Study Guide
1/79
Earn XP
Description and Tags
Inshallah whoever reading this passes with a high grade
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
80 Terms
Oxidation for Group 1
+1
Oxidation for Group 2
2
Oxidation for V
+5 or +4
Oxidation for Fe
+2 or +3
Oxidation for Co
+2 or +3
Oxidation for Cu
+1 or +2
Oxidation for Ag
+1
Oxidation for Zn
+2
Oxidation for Cd
+2
Oxidation for Au
+1 or +3
Oxidation for Hg
2 2+ or 2+
Oxidation for Group 13
+3
Oxidation for Group 14
+4
Oxidation for Group 15
-3
Oxidation for Group 16
-2
Oxidation for Group 17
-1
Mono
1 atom of the element
Di
2 atoms of the element
Tri
3 atoms of the element
Tetra
4 atoms of the element
Penta
5 atoms of the element
Hexa
6 atoms of the element
Hepta
7 atoms of the element
Octa
8 atoms of the element
Nona
9 atoms of the element
Deca
10 atoms of the element
BO33-
Borate
BO23-
Borite
CO32-
Carbonate
CO22-
Carbonite
NO3-
Nitrate
NO2-
Nitrite
PO43-
Phosphate
PO33-
Phosphite
SO42-
Sulfate
SO32-
Sulfite
AsO43-
Arsenate
AsO33-
Arsenite
SeO42-
Selenate
SeO32-
Selenite
TeO42-
Tellurate
TeO32-
Tellurite
SiO44-
Silicate
SiO34-
Silicite
ClO4-
Perchlorate
ClO3-
Chlorate
ClO2-
Chlorite
ClO-
Hypochlorite
BrO4-
Perbromate
BrO3-
Bromate
BrO2-
Bromite
BrO-
Hypobromite
IO4-
Periodate
IO3-
Iodate
IO2-
Iodite
IO-
Hypoiodite
MnO4-
Permanganate
MnO42-
Manganate
CrO42-
Chromate
Cr2O72-
Dichromate
O22-
Peroxide
CN-
Cyanide
OH-
Hydroxide
C2H3O2- or CH3OO-
Acetate
HCO3-
Bicarbonate
S2O32-
Thiosulfate
NH4+
Ammonium
H3O+
Hydronium
Hg22+
Mercury (I)
Ionic Compound Rule
Name of Cation followed by Anion
If the anion is an element, change its ending to…
-ide
If the anion is a polyatomic anion
Write name of the ion
For monoatomic ions, the charge on the ions equals the…
oxidation number
The oxidation numbers assigned to each of the atoms composing a compound or ion should…
sum to equal the total charge of the compound
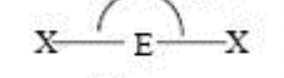
Steric No: 2
Lone Pairs: 0
Electron Geometry: Linear
Molecular Shape: Linear
Degrees: 180°
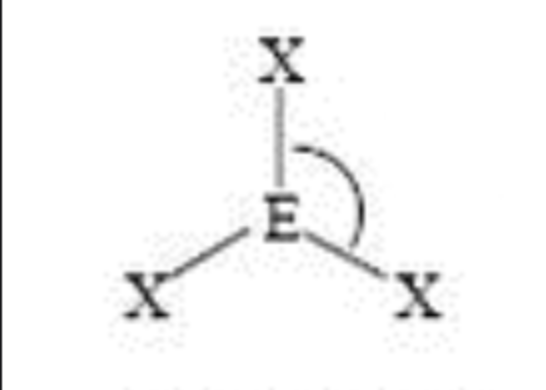
Steric No: 3
Lone Pairs: 0
Electron Geometry: Trigonal Planar
Molecular Shape: Trigonal Planar
Degrees: 120°
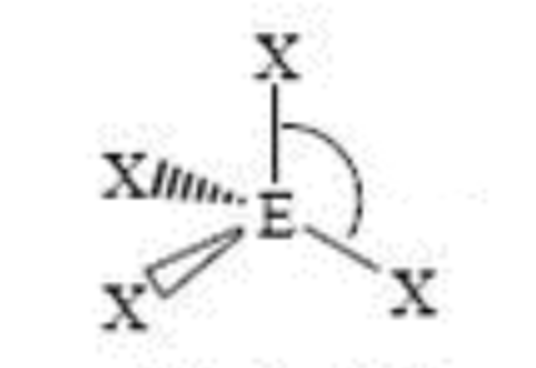
Steric No: 4
Lone Pairs: 0
Electron Geometry: Tetrahedral
Molecular Shape: Tetrahedral
Degrees: 109°
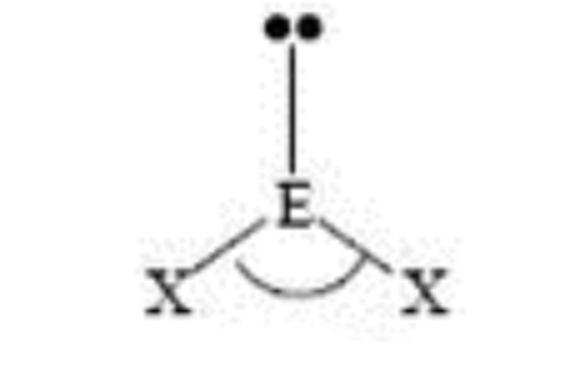
Steric No: 3
Lone Pairs: 1
Electron Geometry: Trigonal Planar
Molecular Shape: Bent
Degrees: <120°
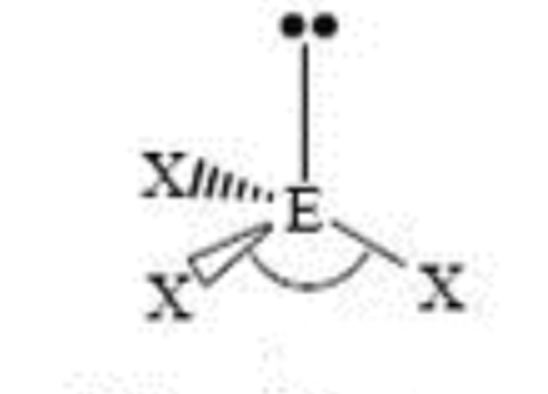
Steric No: 4
Lone Pairs: 1
Electron Geometry: Tetrahedral
Molecular Shape: Trigonal Pyramid
Degrees: <109°
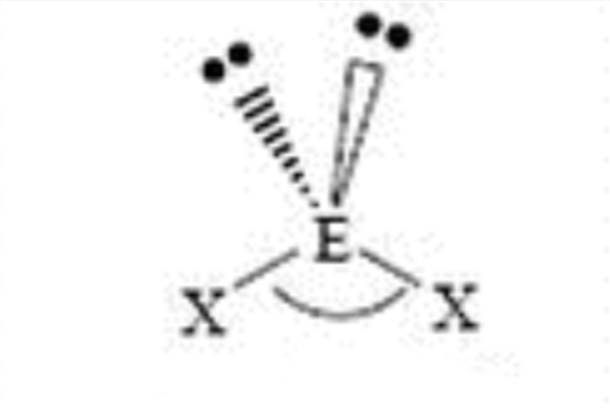
Steric No: 4
Lone Pairs: 2
Electron Geometry: Tetrahedral
Molecular Shape: Bent
Degrees: «109°