Exam 1B Units 4 + 5 - How Do We Characterize & Predict Chemical Processes?
1/28
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
29 Terms
A higher charge means a stronger bond in that molecule
A highly charged molecule reflects what when compared to a weaker charged molecule?
High potential energy means a molecule is weaker in its bond strength
A molecule with a high potential energy reflects what about its strength
A smaller molecule will be stronger and have lower potential energy
A larger sized molecule when compared to a smaller one reflects what about its strength and potential energy?
absorbs energy
When a bond breaks, it _______ energy
releases energy
When a bond forms, it _______ energy
Exothermic
Releasing energy
AA —> AB
Endothermic
Absorbing energy
AB —> AA
Exothermic
Which type of energy reaction is product favored (energetically favored)
Endothermic
Which type of energy reaction is reactant favored
Whether or not the product or reactant has the lower potential energy
Favorability is based on what in energy reactions
Lower temperature
A lower Ep product would be favored at what temp
Higher temperature
A higher Ep product would be favored at what temp
Lower left and upper right quadrant
Which quadrants of a PEC diagram would be temperature dependent
Upper left quadrant
Which quadrant of a PEC diagram would be least favored (doesn’t happen at any temperature)
Less gas —> more gas
Entropically favored
ΔS>0 indicates what?
More gas —> less gas
Entropically UNfavored
ΔS<0 indicates what?
Least stable and therefore more Ep and therefore less of a charge when compared to a molecule of a smaller size.
A largely sized molecule indicates what about its stability?
Increasing the Ep
Increasing entropic stability means what in terms of Ep?
Move to a lower Ep
For a reaction to be energetically favored (∆H<0)
Energetically stable (favored)
Substances with a lower Ep are _____
Entropically stable (favored)
Substances with a higher entropy are _____
Move to a higher number of configurations
For a reaction to be entropically favored (∆S>0)
A product favored reaction would have a higher concentration of products
A reactant favored reaction would have a higher concentration of reactants
Favorable means more likely to happen = requires no energy
Favorability implies what in terms of concentrations when a reaction stops changing
Lower right
Which quadrant of a PEC diagram would be most favored (happens at all temperatures)
Higher entropy and therefore more stable
A higher molar mass indicates what in terms of entropic stability?
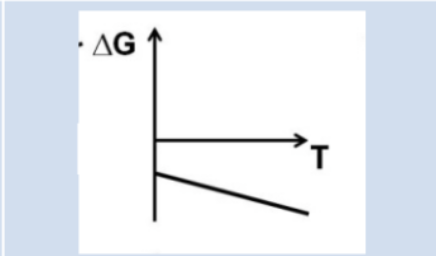
What can we conclude about favorability based on this graph?
At all temperatures, ∆G is always (-)
Always product favored
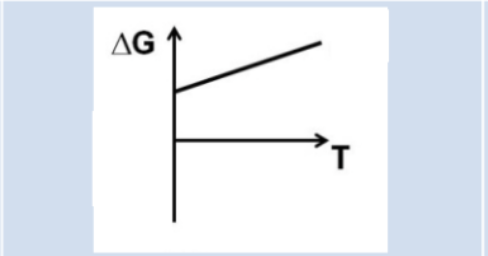
What can we conclude about favorability based on this graph?
At all temperatures, ∆G is (+)
Always reactant favored
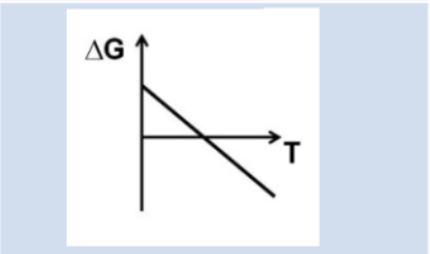
What can we conclude about favorability based on this graph?
At low tempuratures ∆G is (+); at higher temperatures ∆G is (-)
Product favored at high temperature
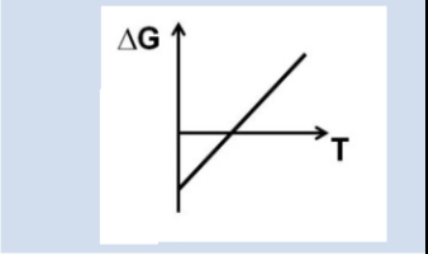
What can we conclude about favorability based on this graph?
At low temperatures ∆G is (-), at higher temperatures ∆G is (+)
Product favored at low temperatures