Fessette - Unit 3: Atomic Structure
1/37
Earn XP
Description and Tags
* the answers for the people are their contributions to atomic theory * flashcards are in order following the notes * sorry for the inconsistent capitalization but I am tired and didn't really know how to format this * good luck and text me if you have any questions! 🫡
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
38 Terms
Democritus (400ish B.C.E.)
The first person to suggest the existence of fundamental particles, also coined the term “atom”
John Dalton (1803-1805)
Had a self-named atomic theory that stated that all matter is composed of atoms (including elements), and that all atoms of a given element are completely identical
Law of Definite Proportions
(Identified by Dalton) Atoms can combine with each other in simple whole number ratios to form compounds
Law of Multiple Proportions
(Identified by Dalton) Atoms can combine in two or more ways to form compounds
J.J. Thomson (1906)
Discovered the electron using a cathode ray tube
Cathode Ray Tube
A glass tube that displays an electron beam that can be manipulated by a magnet, was used to discover electrons
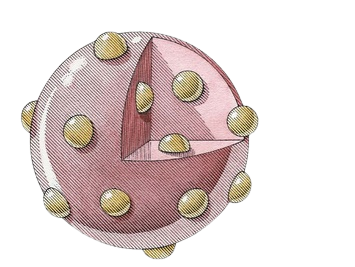
Plum Pudding Model (Thomson’s Model)
Atomic model with electrons embedded in the nucleus
Ernest Rutherford (1911)
Conducted an experiment that proved that atoms had nuclei: Used a radioactive substance to shoot alpha particles at a sheet of thin gold foil surrounded by a film for the particles to display . The particles should’ve passed straight through but few deflected. They had come in contact with the nucleus!
Atomic Nucleus (Rutherford Model)
Atomic model with a positive nucleus with electrons floating around (mostly empty space)
Neils Bohr (1913)
Proposed that electrons orbit the nucleus at principle energy levels (PELs) and that electrons can move between these states through excitation and deexcitation
PEL
principle energy level
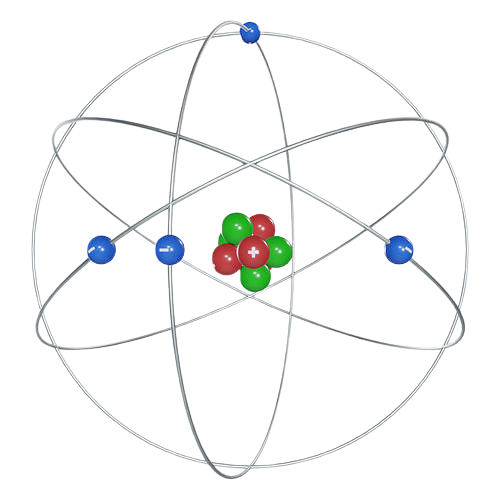
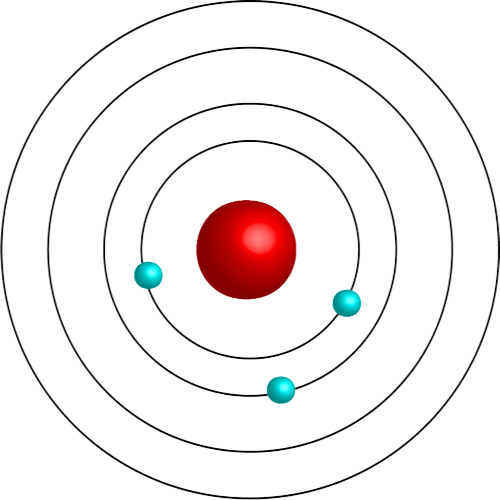
Planetary Model (Bohr Model)
Atomic model with a nucleus and PELs
excited state
when an atom absorbs heat or energy and an electron jumps to a higher PEL
ground state
the lowest PEL/the constant state of the atom
When electrons jump PELs, they release _______.
photons (colored light)
PEL equation
2n² = # of electrons
Erwin Schrödinger (1926)
Proposed that electrons move randomly in orbitals; their locations can never be accurately determined, there’s only a cloud of probability (an orbital) as to where they could be
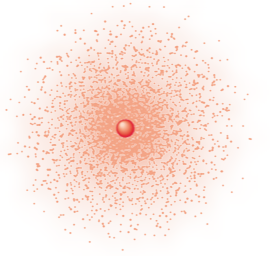
Wave-Mechanical/Electron Cloud (Schrödinger’s Model)
Atomic model that includes a nucleus and a cloud of electron probability around it
AMU
atomic mass unit
What is the mass of a single proton?
1 AMU
What is the mass of a single neutron?
1 AMU
What is the mass of a single electron?
0 AMU
A proton has a _____ charge.
positive
A neutron has a _____ charge.
neutral/nonexistent
An electron has a _____ charge.
negative
Atomic Number
equals the amount of protons in a single element’s atom and determines the type of element an atom is
Mass Number
equals the amount of protons and neutron in a single element’s atom and is the total mass of the singular atom (always a whole number!)
Atomic Mass
the average mass of all atoms of an element in the universe (note that this value is an estimate because we can’t actually measure the mass of every single atom ever)
Valence Electrons
The number of electrons in the outermost PEL of an element’s atom
Kernel Electrons
The number of all the electrons besides the valence electrons
Lewis Structures/Dot Diagrams
symbols that represent the number of valence electrons of an atom
Bonding Sites
locations on a PEL where there is only a single electron that allows atoms to attach or lose their electrons
Isotopes
Atoms of the same element that have different amounts of neutrons
Ions
Atoms of the same element that have different amounts of electrons and now have positive or negative charges
how to solve for atomic mass
(% occurrence converted to a decimal) x (mass number of isotope) = atomic mass of isotope
add the number of results of these (how many depends on how many isotopes) together and sigfig
anion
negatively charged ion (gained an electron)
cation
positively charged ion (lost an electron)
make sure to review notes and stuff and look at the work packet and try to enjoy studying!
woop woop thanks