BIS2A SS1 Week 3
1/80
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
81 Terms
what two processes are found in oxidative phosphorylation?
electron transport and chemiosmosis
electron transport
passage of electrons from one compound to another (can be between complexes) and the capturing that the free energy released by the transfer reaction
what is the energy released from electron transport used for?
oxidation of a compound/reduction of another pumps hydrogen ions/protons, given there is sufficient energy released from the transfer (resulting in an energized membrane)
two types of carriers that aid in electron movement
e-/H+ carriers (usually carry 2 protons, 2 electrons)
e- carriers
e-/H+ carriers
NAD+ox (NADP+ox)/NADH + H+red (NADPH+H+red)
Quinonesox/quinonesred
Flavins (FAD(ox)/FADH2(red))
e- carriers
FeS proteins
cytochromes (have a heme prosthetic group)
NOTE: both of them have an Fe center that can be in +2/+3 oxidation state, depending on if an electron is being carried
primary electron donor, what is the primary electron donors in eukaryotes?
first molecule to donate the electron
in euks, NADH is primary donor (complex I is NADH dehydrogenase)
terminal electron acceptor
the final molecule to receive the electron
aerobic respiration
oxygen is the terminal electron acceptor
combines with 2 protons to make water (waste product)
anaerobic respiration
another compound besides oxygen is the terminal electron acceptor
carriers
transfer of electrons usually involves one or more intermediates between donor and acceptor
donors usually have a lower reduction potential than the acceptors
net energy change
difference between the reduction potential of the primary donors and the terminal acceptor
also the change in free energy
why is the ETC done in steps?
to release packets of energy to pump the protons
too much energy released at once can harm the cell
what is an energized membrane/electrochemical gradient used for?
to make ATP in ETC and to move other compounds into the cell (like proton symporters)
chemiosmosis
formation of energized membrane that can do work, in this case form ATP
ATP formation is reversible
efficiency of the ETC chain
dependent on the difference in reduction potential from the primary electron donor to the terminal electron acceptor —> larger E0’ drop = more nrg to pump proton
more H+ translocated/e- donated = more efficient
oxidase vs reductase
oxidase (oxygen involved) — cytochrome oxidase (complex 4 of ETC) reduces oxygen
reductase = no oxygen involved
why was using oxygen as the terminal electron acceptor so revolutionary?
because oxygen has a high tendency to be reduced, it can generate a large ∆E0’ drop, which increases ATP production
efficiency of aerobic vs anaerobic respiration
aerobic respiration is generally more efficient than anaerobic respiration due to the usage of O2 as a terminal electron acceptor
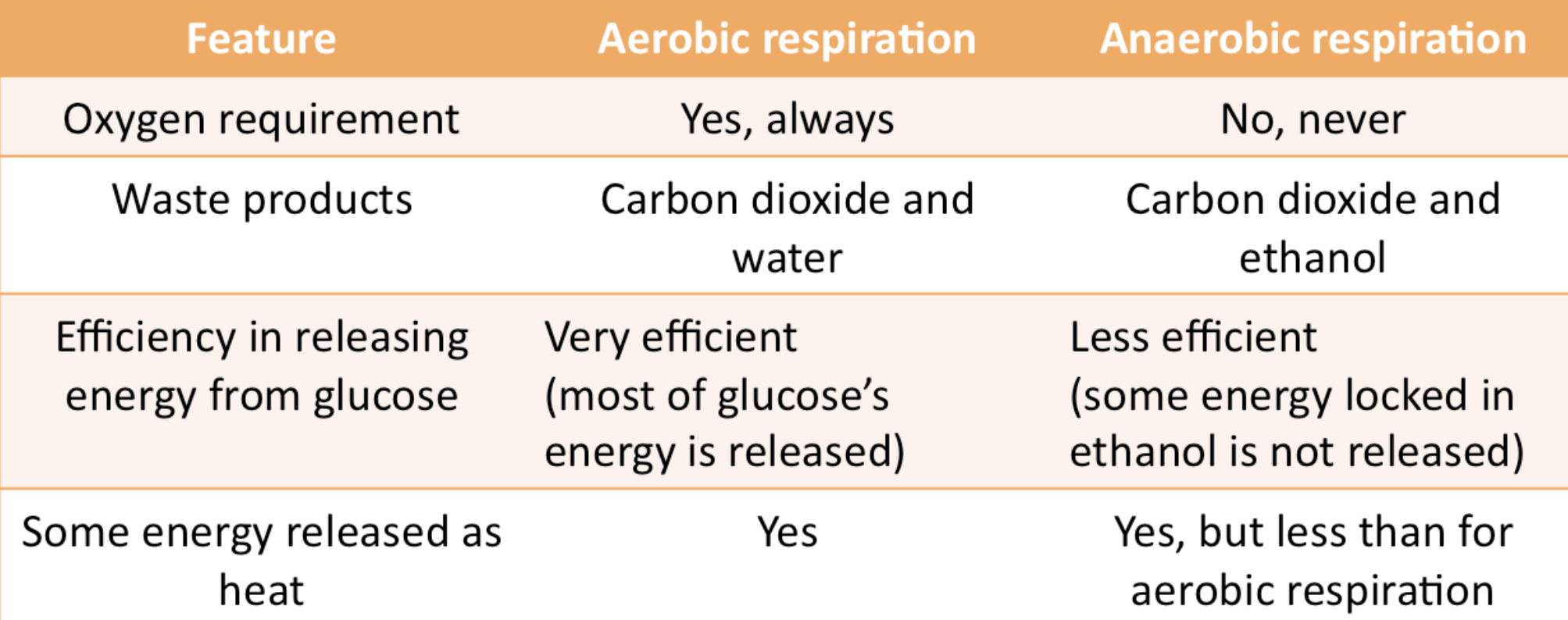
complex II of ETC
oxidation of FADH2 by succinate dehydrogenase does not pump any protons across the membrane because the energy drop is not sufficient
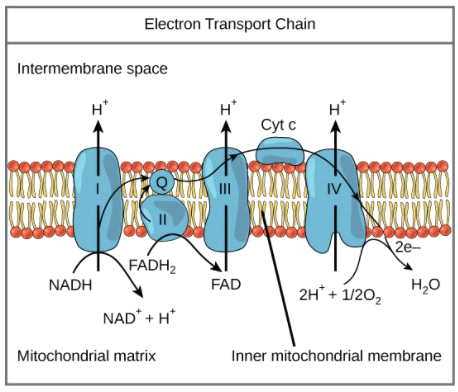
complex III of ETC
cytochome c reductase; can pump protons across membrane
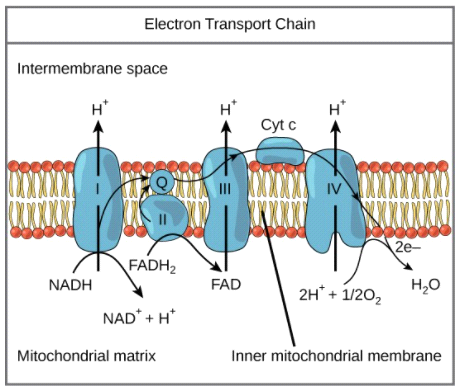
complex IV of ETC
cytochome c oxidase; can pump protons across as well; reduces oxygen (the terminal electron acceptor), which combines with 2 protons to make water
what 4 things can happen when an electron gets excited?
energy dissipated as heat as electron relaxes
energy dissipated at florescence as electron relaxes
energy transferred to neighboring molecule (not 100% efficient)
energy changes reduction potential so it can become e- donor (coupled to redox)
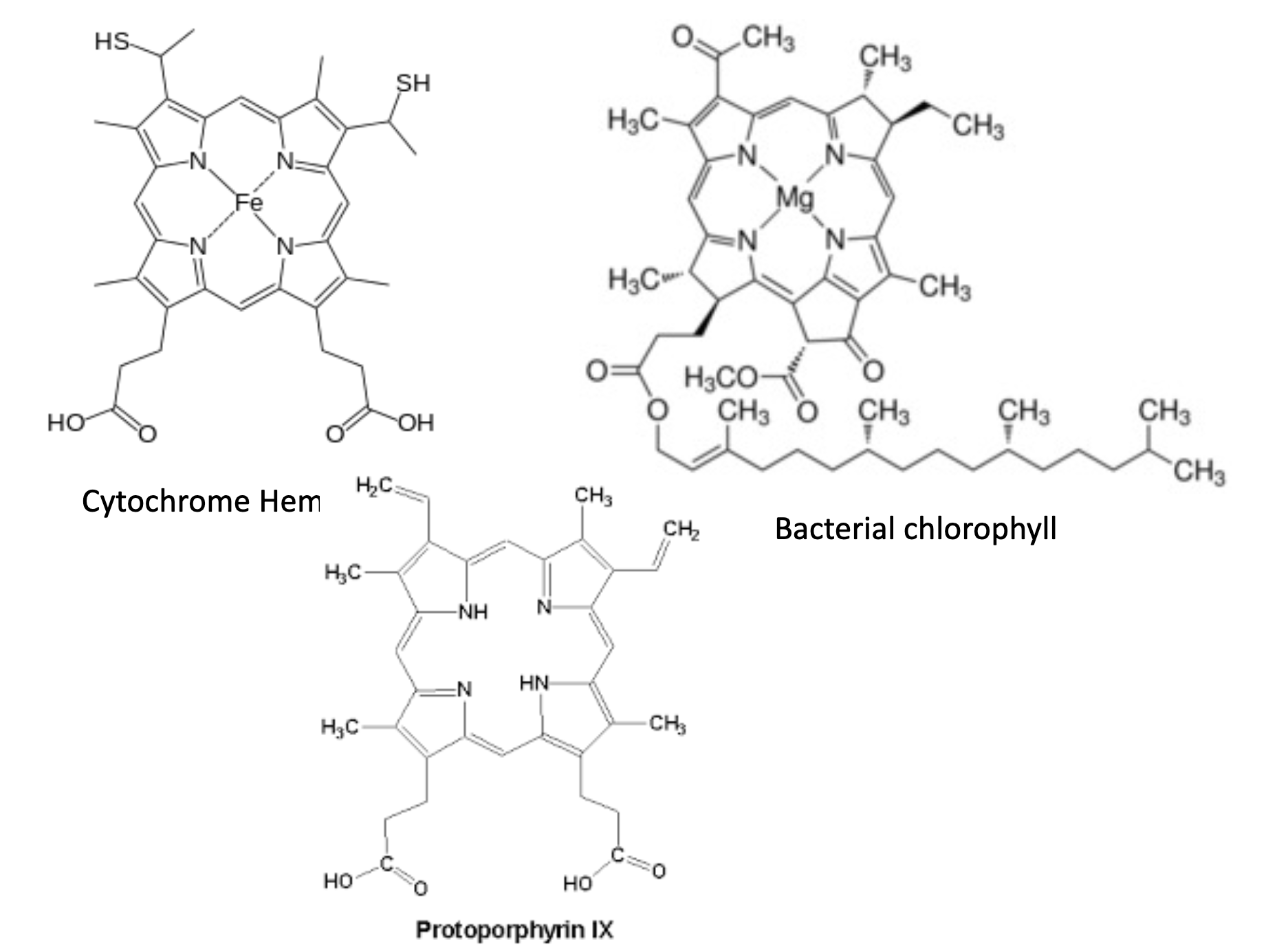
protoporphryin IX, cytochome heme and bacterial chlorophyll
protoporphyrin IX is the precursor to heme/bac. chlorophyll
heme has an iron center
bac. chlorophyll has a magnesium center and a lipid tail (anchors chlorophyll in membrane)
anoxygenic photosynthesis
does not release oxygen/doesn’t use water as a source of electrons; found in 2 forms (cyclic and noncyclic photophosphorylation)
cyclic photophosphorylation
electron that was excited and moved through the ETC ends up back at the reaction center it started at
generates ATP only
noncyclic photophosphorylation
electron that was excited ends up being donated to NADP+ to form NADPH
needs a source of electrons from reduced compounds (like H2S)
NADH vs NADPH
NADH is used in catabolic processes
NADPH is used in anabolic processes
chlorophyll a
has a greater reduction potential than oxygen, it can oxidize water as a electron source (releases oxygen as waste)
oxygenic photosynthesis
uses water as electron source and chlorophyll a to capture light energy to excite electron; can pump protons across the membrane to generate ATP AND can reduce NADP+ at the end of this
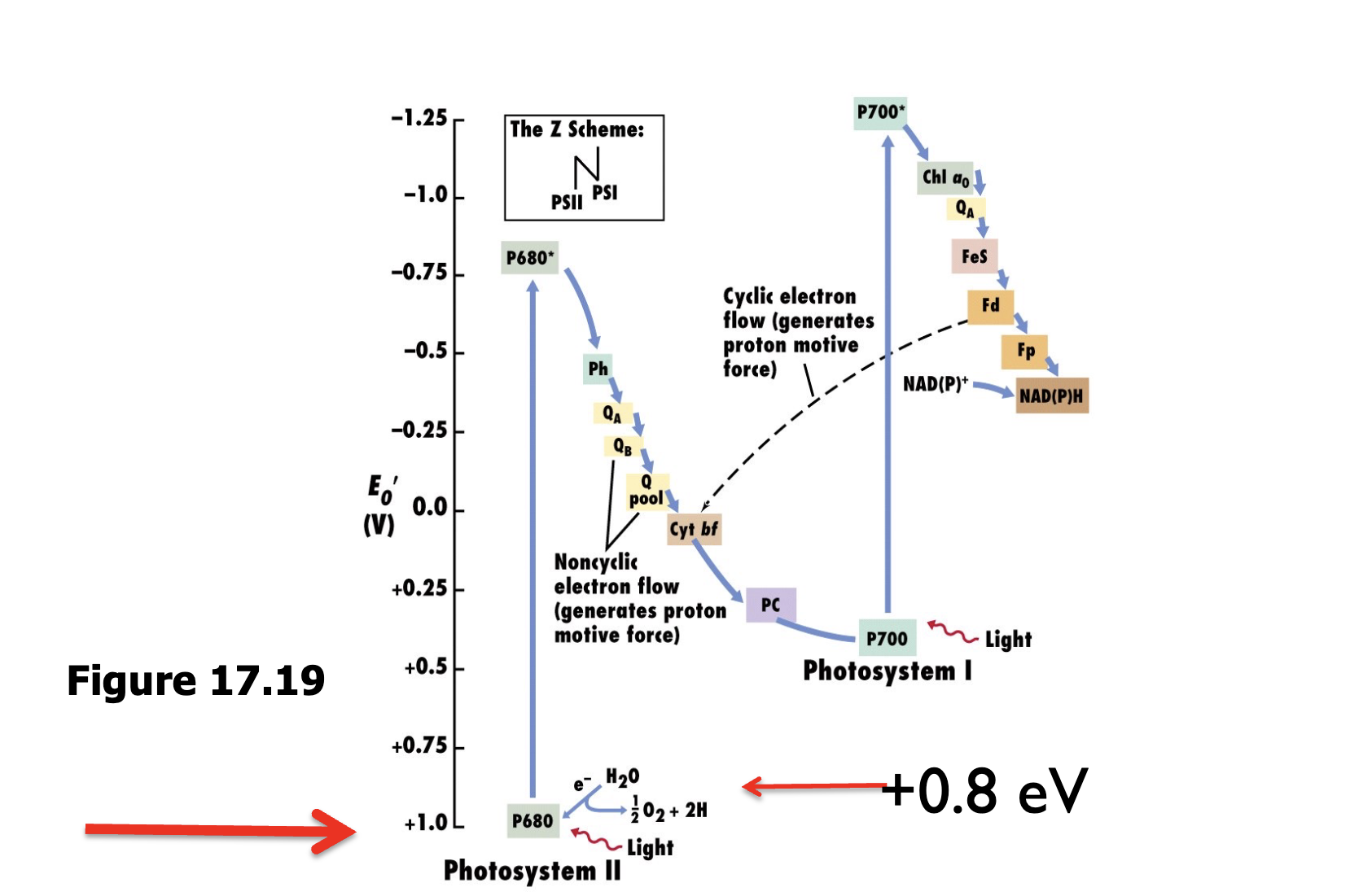
Z scheme
starts as PSII, electron gets excited and move down through the ETC (pumps H+ across the membrane to generate proton motive force)
electron goes to PSI, gets reexcited and moves through another ETC which generates NADPH using NADP reductase
anoxygenic vs oxygenic photosynthesis
what do CH bonds tell us about how reduced a compound is?
more CH bonds = more reduced
ex. CO2 (which has no CH bonds) is the most oxidized form of carbon available
what is an organic compound?
a compound that contains CHO and is made by another organism (that is alive)
heterotrophs
make carbon building blocks from transforming small organic compounds
autotrophs
make carbon building blocks by reducing the highly oxidized carbon source CO2 (not organic)
catabolism
all the processes in a cell that generate energy (break down compounds)
anabolism
all the processes that build compounds
metabolism
all the processes in a cell — catabolism + anabolism
central metabolism, what processes are included in central metabolism
the metabolic processes all cells need to do all other processes from the catabolic breakdown of glucose — creates precursors
includes glycolysis, pyruvate oxidation, TCA cycle, pentose phosphate pathway (PPP)
what 3 things do cells need to build other cells (comes from central metabolism)?
small molecules for anabolic processes (precursors)
reducing power (like NADH/NADPH)
energy (like ATP)
steady state
cells do not have to change physiology to do processes (no change in environment = no change in procsses)
OR a constant/equal concentration of metabolites
metabolic hypothesis
metabolism evolved as a means of generating small carbon molecules for anabolic reactions (not as a means to generate energy)
the 12 major precursors created by central metabolism that are required to build cell + what are they produced from
glycolysis - glucose 6-phosphate, fructose 6-phosphate, triose-P, 3-phospho-glycerate, phosphoenolpyruvate, pyruvate
PPP - pentose 5-phosphate, erythrose 4-phosphate
pyruvate oxidation: acetyl-CoA
TCA: alpha-Ketoglutarate, oxaloacetate, succinyl-CoA
13th one in gram negative: sedoheptulose (PPP)
glycolysis
oxidation of glucose into pyruvate
net results of glycolysis
2 ATP from SLP (2 consumed; 4 produced)
2 NADH + H+
produces 6 precursors
2 pyruvates (3 carbon) from one glucose
which key reaction in glycolysis is not reversible
phosphoenolpyruvate —> pyruvate (catalyzed by pyruvate kinase)
the 2 fates of pyruvate
fermentation to regenerate NAD+
respiration (produce acetyl-CoA)
pyruvate oxidation
each pyruvate is decarboxylated and oxidized to acetyl-CoA
produces 1 CO2 and 1 NADH per pyruvate
net results of glycolysis and pyruvate oxidation
2 ATP from SLP
4 NADH + H+ (2+2)
7 precursors (6+1)
2 acetyl-CoA/oxidized glucose
what happens to acetyl-CoA?
goes to citric acid/TCA/Krebs cycle; completely oxidized to CO2
citric acid cycle
2 carbon acetate enters; is a cycle because it regenerates oxaloacetate (which acetyl gets fixed onto)
produces 3 major precursors: alpha-ketoglutarate, succinyl CoA, oxaloacetate
3 NADH; 1 FADH2; 1 SLP; 2 CO2 per pyruvate
what are the end results of the oxidation of glucose (central metabolism)?
6 CO2
10 NADH (2+2+6)
2 FADH2 (citric acid)
4 ATP via SLP(2+2)
10 precursors (6+1+3)
what 2 key substrates are not produced from glycolysis, pyruvate oxidation, TCA?
pentose 5-phosphate
NADPH + H+ for anabolic reactions
oxidation of glucose in the pentose phosphate pathway
3 glucose 6-phosphate —> 3 6-phosphogluconate (produces 3 NADPH + H+)
3 6-phosphogluconate —> 3 ribulose 5-phosphate (3 NADPH + H+ and 3 CO2)
rearrangments of the pentose 5-phosphate
3 pentose-P —> 2 hexose-P (F6P) + 1 triose-P (G3P)
net results of pentose pathway (per 3 glucose)
6 NADPH + H+
2 precursors (pentose and erthyrol P intermediates)
3 CO2
1 G3P
2 hexose-P
what is the driving force of these reactions?
concentration of metabolites in environment (want to reach equilibrium)
what is one caveat of central metabolism and the 12 central compounds?
we need the enzymes for these reactions (humans don’t have them)
we need to get from outside that are readily available (ex. 9 essential amino acids)
Calvin cycle
RuBP is fixed onto 3 CO2 by rubisco and then broken into 2 3-carbon intermediates
takes 2 turns of the calvin cycle to get 2 G3P (the rest of the 10 G3P regenerate 5 carbon RuBP)
what is used in calvin cycle
9 ATP and 6 NADPH per 3 CO2 (therefore calvin cycle is reductive)
the link between calvin cycle and photophosphorylation
light induces pH changes that activate calvin cycle
light allosterically modulates enzyme activity by reducing disulfide bonds to activate calvin cycle enzymes
other ways to fix CO2
reductive citric acid cycle
reductive acetyl-CoA pathway
hydroxypropionate pathway
why is CO2 useful?
gets rid of excess carbon
starvation enzymes can bypass decarboxylations to conserve CO2 to make sugars
biomass
amount of carbon in an organism
tree biomass from atmosphere
we exhale CO2 at night (biomass lost overnight)
gluconeogenesis
formation of glucose form cellular substrates in low glucose conditions
need to have alternate pathways to make glucose (ex. PEP to pyruvate is irreversible; use alternative reaction that costs 2 ATP and 2 GTP)
reductive TCA
fixes 2 carbon molecules to make acetyl-CoA in organisms without electron transport chains; allows organisms to make 4 of 12 essential compounds
reactions run in reverse, costs NADPH to reduce CO2
phosphoanhydride bonds
high energy bonds between phosphates that can be hydrolyzed to release energy
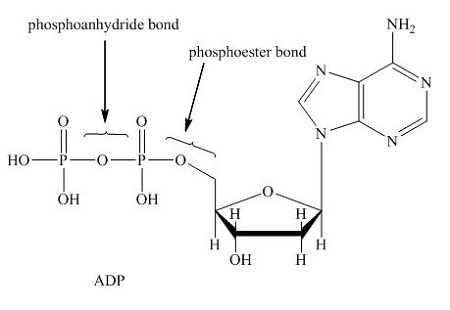
how to extract energy from ATP
hydrolyze phosphoanhydride bonds
phosphorylation
adding a phosphate group; used to regenerate ATP
what sugar do hexoses flow to?
glucose!
can hexoses, trioses, pentoses enter at different points of central metabolism?
yes!
for example, a pentose or erythrose sugar can enter in the pentose phosphate pathway
trioses can feed into G3P
deamination reactions
removal of amine group from a molecule
what are deamination reactions used for?
converting amino acids into intermediates of central metabolism by deamination reactions
usually in organisms that eat protein (like humans)
when do amino acids feed into central metabolism?
TCA/end of glycolysis
what’s the problem with getting intermediates from amino acids?
you can only do these reactions if you have the genes to make the enzymes
4 ways to control carbon flow
ATP expended to push reactions
keep product concentrations low (done automatically in pathways)
metabolite concentrations near Km (reversible reactions)
feedback regulation (allosteric regulations)
feedback regulation
small metabolites can allosterically regulate enzymes in that pathways
enzymes are sensitive because working at Km
do substrates activate or inhibit?
activate
do products activate or inhibit?
inhibit
what are the two major ratios can control pathways
NADH: NAD
ATP: ADP and AMP
buildup of NADH/ATP (products) will inhibit
buildup of ADP+AMP/NAD will activate