Organic Chemistry Common pKa Values
0.0(0)
0.0(0)
Card Sorting
1/26
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
1
New cards
pKa = 25
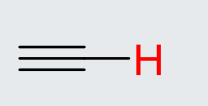
C2H2 (Alkyne)
2
New cards
pKa = 50
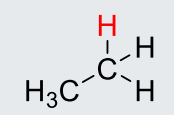
CH3CH3
3
New cards
pKa = 44
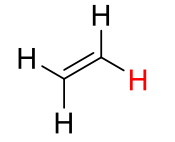
C2H4
4
New cards
pKa = 38
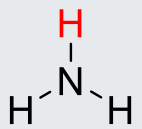
NH3
5
New cards
pKa = 16-18
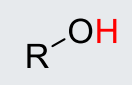
Alkyl-OH
6
New cards
pKa = 15.7
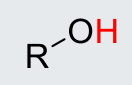
H2O
7
New cards
pKa = 9
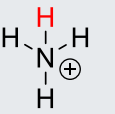
NH4+
8
New cards
pKa = 7
H2S
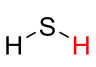
9
New cards
pKa = -9
whatever that is
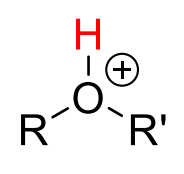
10
New cards
pKa = -2
H3O+
11
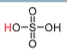
New cards
pKa = -9
H2SO4

12
New cards
pKa = -9
HBr
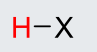
13
New cards
pKa = -10
H-Iodine
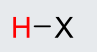
14
New cards
pKa = -7
H-Cl
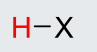
15
New cards
pKa = 3
H-F
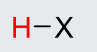
16
New cards
pKa = -8
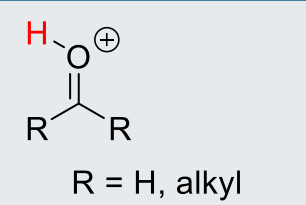
Alykl2C-OH+ or CH2-OH+
17
New cards
pKa = -2
H3O+
18
New cards
pKa = -3
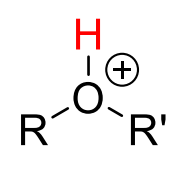
Alkyl-H2O+
19
New cards
pKa = -4
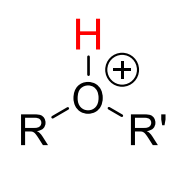
Alkyl2-OH+
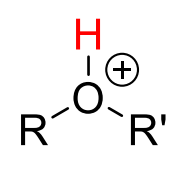
20
New cards
pKa = 5
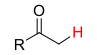
Alkyl-CO2H or CO2H2 (carboxylic acid)
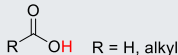
21
New cards
pKa = 10
Phenol
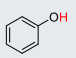
22
New cards
pKa = 15
Ring with H2….
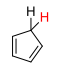
23
New cards
pKa = 9
Fake anhydride molecule (with R = H, alkyl)
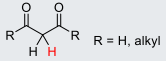
24
New cards
pKa= 9
H-CN
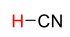
25
New cards
pKa = 17
C2OH2
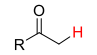
26
New cards
pKa = 19
Alkyl-C2OH (Ketone kinda)
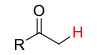
27
New cards
pKa = 25
C2O2H