Proton gradient intro and energy
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
In the electron transport chain (ETC), you can track which components are oxidized or reduced by…
observing how they interact with light (optical spectra).
How does optical spectra work?
Scientists use spectrophotometry to detect where a blockage is in the ETC by checking the redox state of each carrier.
Many ETC carriers (like cytochrome c) change color depending on whether they are oxidized (Fe³⁺) or reduced (Fe²⁺).
What happens to ETC carriers before the point of obstruction?
They become reduced, because they receive electrons but cannot pass them forward.
What happens to ETC carriers after the point of obstruction?
They remain oxidized, because no electrons are reaching them.
What is the proton motive force (PMF)
Proton motive force is the energy created by having more protons on one side of the mitochondrial membrane than the other. This energy is used to make ATP.
What are the two components of the proton motive force?
1) Chemical potential energy from the H⁺ concentration difference (bc they are pumped into the IMS highering the H+ outside the matrix)
2) Electrical potential energy from charge separation.
due to proton pumping the IMS becomes positively charged and teh matrix becomes negatively charged
What enzyme uses the proton motive force to make ATP?
ATP synthase
What does pumping protons across the membrane cause
Creates a pH difference (the outside becomes more acidic). → chemical
Creates a voltage difference (the outside is more positive). → electrical
Every time 1 mole of H⁺ is pumped across the membrane, the cell stores about _____ of energy.
20Kj of energy
5 from pH difference
15 from voltage difference
Since 10 protons are pumped per NADH, that's about _____ stored in the gradient.
200kj (1proton=20kj ratio)
What does chemiosmotic theory explain?
How the energy from electron transport is used to create a proton gradient that powers ATP production.
What happens as protons flow through ATP synthase?
The enzyme rotates and synthesizes ATP from ADP and Pi.
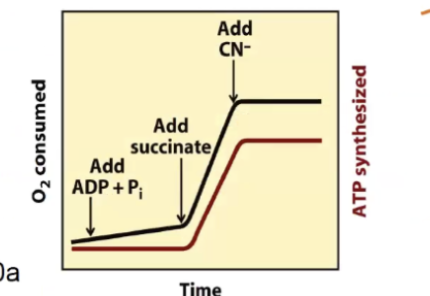
Proof of chemiosmotic theory
An inhibitor of electron transfer will inhibit both ATP synthesis - it levels off
Inhibition of ATP synthase also blocks the electron trasnport chain (if its blocked by drug oligomycin for example, ETC levels off)
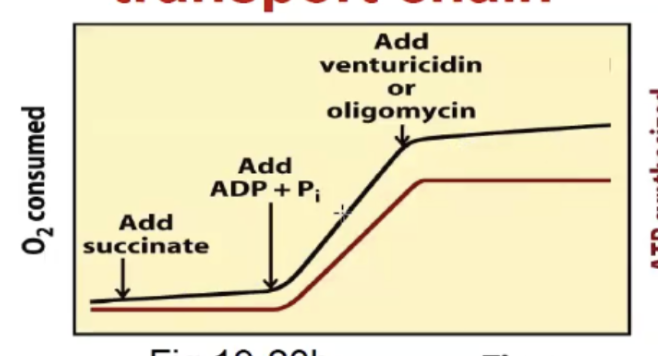
Are the ETC and ATP synthesis coupled?
Yes, the ETC pumps protons → builds a gradient → ATP synthase uses that gradient to make ATP.
But if something allows protons to bypass ATP synthase, the system becomes uncoupled:
Electron transport still runs (NADH is oxidized, O₂ is reduced).
ATP production stops — because the proton gradient is being dissipated without going through ATP synthase.
What could cause the ETC and ATP synthesis to uncouple?
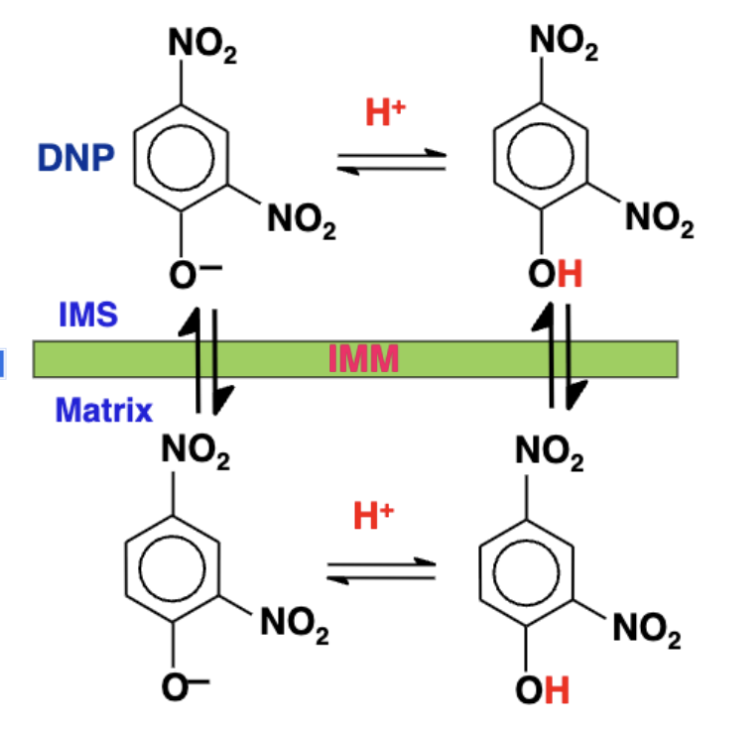
Dinitrophenol (DNP)
it passes through membranes grabs proteons in the IMS carries protons back into the matrix (not through ATP synthase)
as a result teh proton gradient collapses, ATP synthase cant function
ETC keeps running, but no ATP is being made,
2,4-DNP mechanism
DNP⁻ grabs a proton in the intermembrane space (IMS), becoming DNP-H (neutral).
DNP-H crosses the inner mitochondrial membrane (IMM) into the matrix (it’s neutral and hydrophobic enough to move freely).
In the matrix, DNP-H releases the proton (H⁺) → becomes DNP⁻ again.
DNP⁻ crosses back to the intermembrane space due to its delocalized charge (it still acts a bit like a hydrophobic molecule because of its aromatic ring).
The cycle repeats, collapsing the proton gradient.
ATP synthase can no longer make ATP because there's no gradient.
Energy is lost as heat — not stored as ATP.
causes body to overheat leading to death
2,4-DNP structure
recognize it!
Rotenone blocks the transfer of electrons from NADH to ubiquinone in tightly coupled mitochondria. Under these circumstances,
A. Addition of NADH will lead to the synthesis of ATP at a P/O ratio of 1.5
B. Electron transfer to O2 will continue if succinate were added to the mixture
C. Electron transfer from FADH2 to ubiquinone will cease and Q will remain in an oxidized state
D. Electron transfer from NADH will cease, but O2 consumption will continue
B
Succinate feeds electrons into Complex II, not Complex I.
Rotenone only blocks Complex I, so Complex II (succinate dehydrogenase) still passes electrons to CoQ, and the ETC continues from Complex III → IV → O₂.
chemiosmosis
Electron transport chain (ETC) pumps H⁺ ions (protons) from the mitochondrial matrix into the intermembrane space (IMS).
⚡ This creates a high concentration of protons in the IMS → a proton gradient is formed.
🔋 This gradient stores potential energy, also called the proton motive force (PMF).
🚪 Protons want to go back into the matrix (down their gradient), but they can only do this by passing through ATP synthase, like water through a turbine.
🌀 As protons flow through ATP synthase, it spins and produces ATP from ADP + Pi.
Structure of ATP synthase
F1 → located in the matrix, responsible for synthesis
F0 → Embedded in the inner mitochondrial membrane, forms a channel for protons to flow through
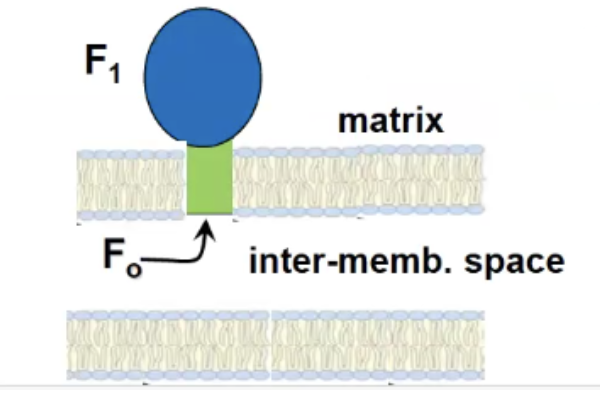
so protons floe up the greenm from the IMS go to blue and ATP synthase spins and makes ATP
How many subunits does F1 have?
9 in total
3 alpha, 3 beta (meaning three catalytic sites, alpha beta pairs) each alpha is idential each beta is identical)
1 gamma,
1 delta
1 epsilon
How may atp can ATP synthase make at a time?
3 because tehre are three catalytic sites
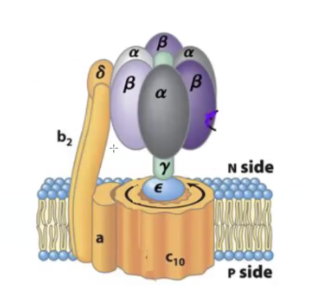
F1 gamma, epsilon, delta subunits
gamma forms the stalk
base is epsilon - which is attaches F1 to F0
delta is attached to F0
F0 functional domain
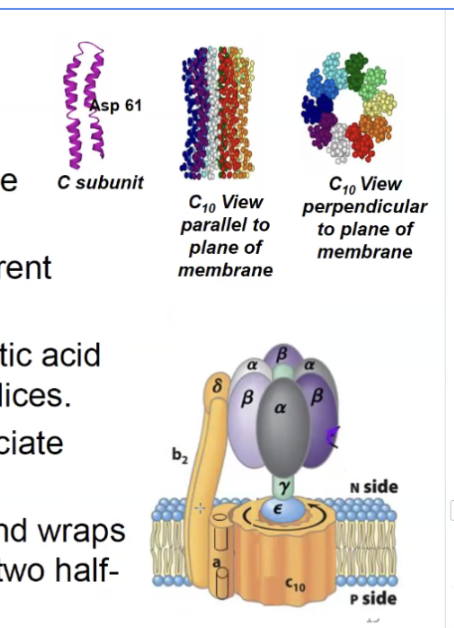
F0 is the yellow part except delta
forms the rotating motor powered by the proton gradient
made of an a subunit which connects c and b subunits, b2 subunits which connect the motor to F1
and 10 c subunits that form the ring
What is the equilibrium constant for ATP formation inside ATP synthase?
Close to 1, meaning the reaction is almost balanced (free energy change near zero).
Why does ATP synthase need energy to release ATP?
Because ATP binds very tightly to the enzyme’s active site after being made
atp doesnt cost that much energy to bring adp and phosphate together, once we join them together we need to releasing them out with some energy that's where it becomes a challenge
Where does ATP synthase get energy to release ATP?
From the proton gradient (proton motive force) driving conformational changes.
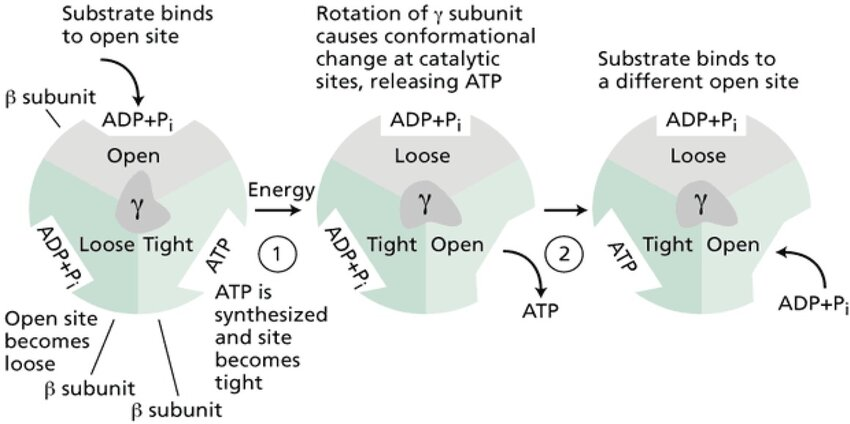
What are the three conformations of ATP synthase’s catalytic sites?
Loose (bind ADP + Pi), Tight (form ATP), and Open (release ATP).
What is rotational catalysis in ATP synthase?
The process where ATP synthase rotates, causing its active sites to cycle through conformations that synthesize and release ATP.
How does ATP synthase release ATP despite the high energy barrier?
By rotating and changing the shape of its catalytic sites, mechanically pushing ATP out.