Biochem general
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
67 Terms
benzene ring functional group name
Phenyl Ph
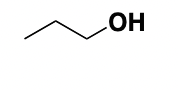
n-propanol
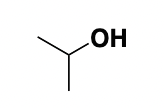
i-propanol
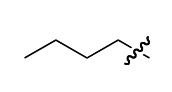
n-butyl
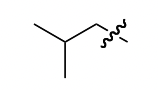
i-butyl
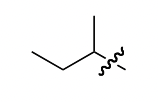
sec-butyl
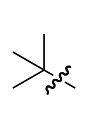
tert-butyl
-OH
Hydroxyl
R-OH
alcohol
C=C
alkene
C≡C
alkyne
R-O-R
Ether
R-NH2
Amine
R-NO2
Nitro compound
R-X
alkyl halide
R-F
flouro
R-Cl
chloro
R-Br
bromo
R-I
iodo
C=O
Carbonyl
R-CHO
aldehyde
R1-CO-R2
ketone
R-CO2H
carboxylic acid
R1-CO2-R2
Ester
R-CONH2
Amide
R-CN (C≡N)
Nitrile/cyanide
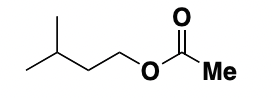
group next to Me
ester
functional group
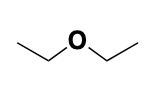
ether
functional group
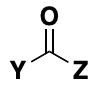
carbonyl
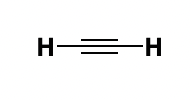
ethene
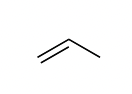
propene
Lowest energy conformation of cyclohexane
chair
unstable, higher energy conformation of cyclohexane
boat
non identical molecules with the same molecular formula
Isomers
Non-identical molecules that vary in the “connectivity” of their atoms with the same molecular formula
constitutional isomers
Non-identical molecules with the same consitution
stereoisomers
Molecule that is superimposable on its mirror image
achiral
molecule that is not superimposable on its mirror image
chiral
two stereoisomers that cannot be superimposed on each other
enantiomers
Cahn-Ingold-Prelog rule clockwise
R
Cahn-Ingold-Prelog rule counterclockwise
S
nucleophiles
electron rich lewis bases
electrophiles
electron deficient lewis acids
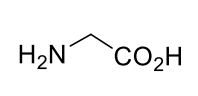
Glycine Gly G
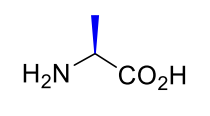
Alanine Ala A
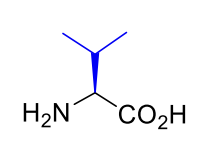
Valine Val V
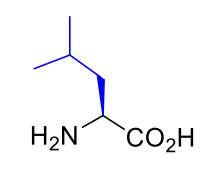
Leucine Leu L
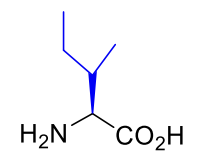
Isoleucine Ile I
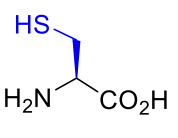
Cysteine Cys C
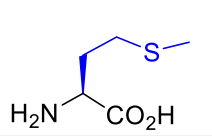
Methionine Met M
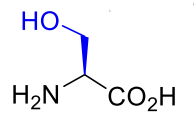
Serine Ser S
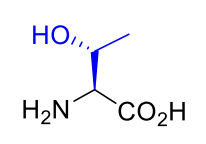
Threonine Thr T
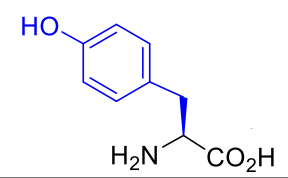
Tyrosine Tyr Y
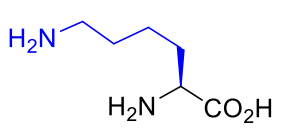
Lysine Lys K
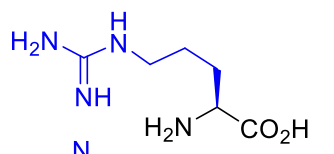
Arginine Arg R
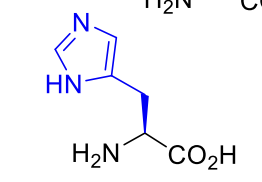
Histidine His H
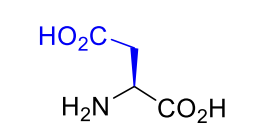
Aspartic acid Asp D
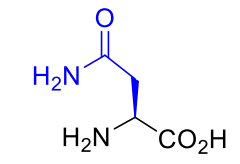
Asparagine Asp N

Glutamic Acid Glu E
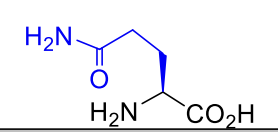
Glutamine Gln Q
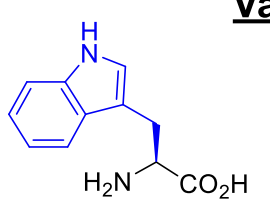
Tryptophan Trp W
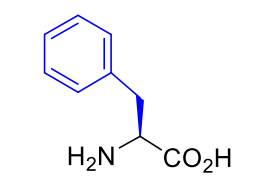
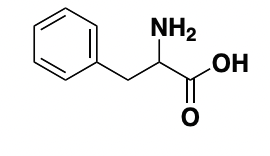
Phenylalanine Phe F
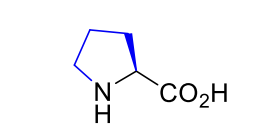
Proline Pro P
aldose carbonyl location
end
ketose carbonyl type
ketone
Markovnikov’s rule
H adds to least substituted carbon
Oxymercuration-reduction reactants needed
1. Hg(OAc)2, H2O/THF 2. NaBH4, EtOH