Elimination Reactions of alcohols
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
To produce halogenoalkanes, alkanes undergo a(n)...
free-radical substitution reactions with halogens
When alcohols undergo elimination reactions, they produce…
alkenes and water
To carry out elimination reactions of alcohols, we use…(2)
a concentrated acid catalyst
heat the mixture
What is the name of this type of reaction?
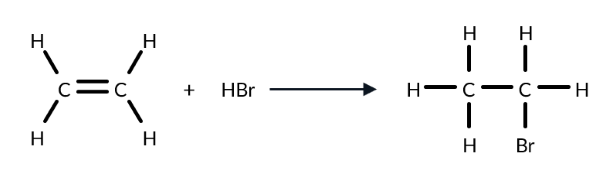
This reaction involves the breaking of 1 pi bond and the forming of 2 sigma bonds, so it’s an addition reaction. As HBr acts as an electrophile, it’s specifically an electrophilic addition reaction
From which carbon(s) could hydrogen be removed in the following reaction?
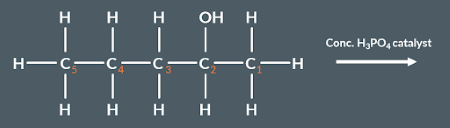
1 and 3
When pentan-2-ol undergoes an elimination reaction, we can produce…
Z-Pent-2-ene
E-Pent-2-ene
From which carbon could hydrogen(s) have been removed in the following elimination reaction?
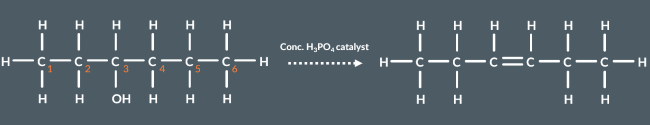
4
From which carbon(s) could hydrogen be removed in the following elimination reaction?
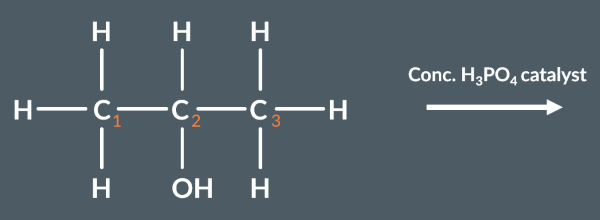
1 and 3
When we write equations for the elimination reaction of halogenoalkanes, we write which type of formula…
the structural formula of the halogenoalkane
the molecular formula of the alkene when it doesn’t have position isomers
the structural formula of the alkene when the alkene has position isomers
Predict which structural isomers could form during this elimination reaction.
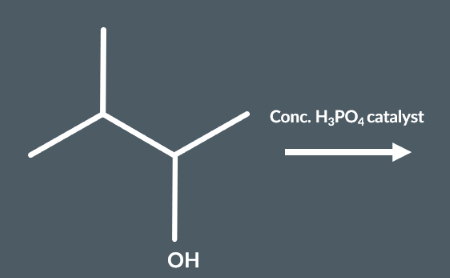
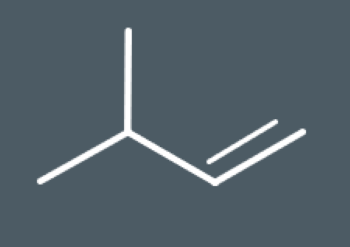
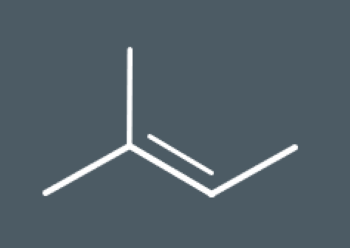
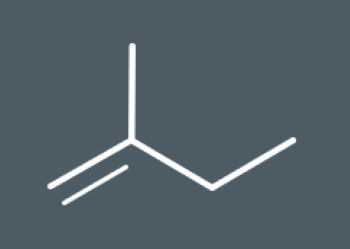
During elimination reactions, secondary carbocation intermediates may undergo rearrangement reactions. During this reaction, the carbocation rearranges its atoms to form a…
tertiary carbocation