Chemistry A LEVELS
1/66
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
67 Terms
Atoms
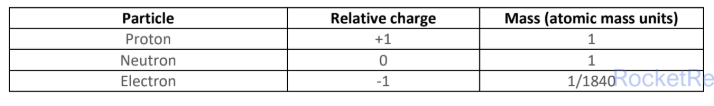
consists of small, dense positively charged nucleus consisting of protons and neutrons, surrounded by negatively charged electrons
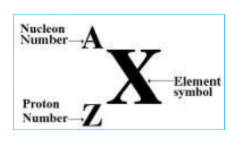
Proton number (atomic number) - is the number of protons in the nucleus of an atom
nucleon number (mass number) - is the total number of protons and neutrons in the nucleus of atom
the mass of an atom is concentrated in the nucleus, because the nucleus contains the heaviest subatomic particle
the electrostatic charge between positive nucleus and negatively charged electrons holds an atom together
A beam of protons, neutrons and electrons moving at the same velocity
o Protons – less and slow deflection towards negatively charged plate
o Neutron – no deflection, go straight
o Electrons – more and faster deflection towards positively charge plate
Atomic Radius (measure of the size of atoms)
o As we move from left to right of a periodic table, the atomic radii decrease as nuclear charge increase so electrons are pulled closer to the nuclei so the atom becomes smaller.
the larger the nuclear charge the greater the pull if the nuclei on the electrons
o As we move down a group, atomic radii increase as electrons are getting added up to new
shells
Ionic radius (measure of size of ions)
o Increase with increasing negative charge – more electrons gained, weak force holding electrons and nuclei – larger ionic radii
o Decrease with increasing positive charge – electrons lost, more force, electrons pulled towards the nuclei – smaller ionic radii
Isotopes
They are atoms of the same element that contain the same number of protons and electrons but a different number of neutrons
the symbol for an isotope is the chemical symbol (or word) followed by a dash then the mass number (e.g. carbon-12 and carbon-14)
isotopes of the same element display the same chemical characteristics, as they have the same number of electrons.
electronic configuration
The arrangement of electrons in an atom. Electrons are arranged around the nucleus in principal quantum shells
PRINCIPLE QUANTEM SHELLS
used to number the energy levels of quantum shells
the higher the principal quantum number, the higher the energy of the shell, greater distance it is from the nucleus
> n=1: up to 2 electrons
> n=2: up to 8 electrons
> n=3: up to 18 electrons
> n=4: up to 32 electrons
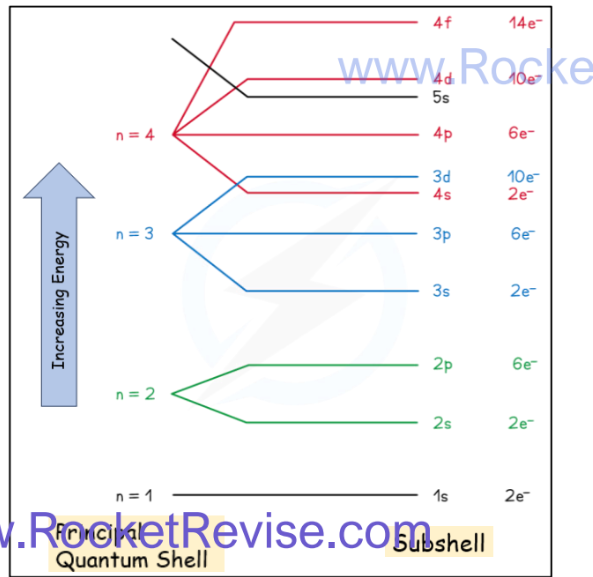
Subshells
o The principal quantum shells are split into subshells which are given the letters s, p and d
o The energy of the electrons in the subshells increases in the order s < p < d
o The order of subshells appears to overlap for the higher principal quantum shells as seen in the diagram below:
Subshell
Contain one or more atomic orbitals. Each atomic orbital can be occupied by a maximum of two electrons.
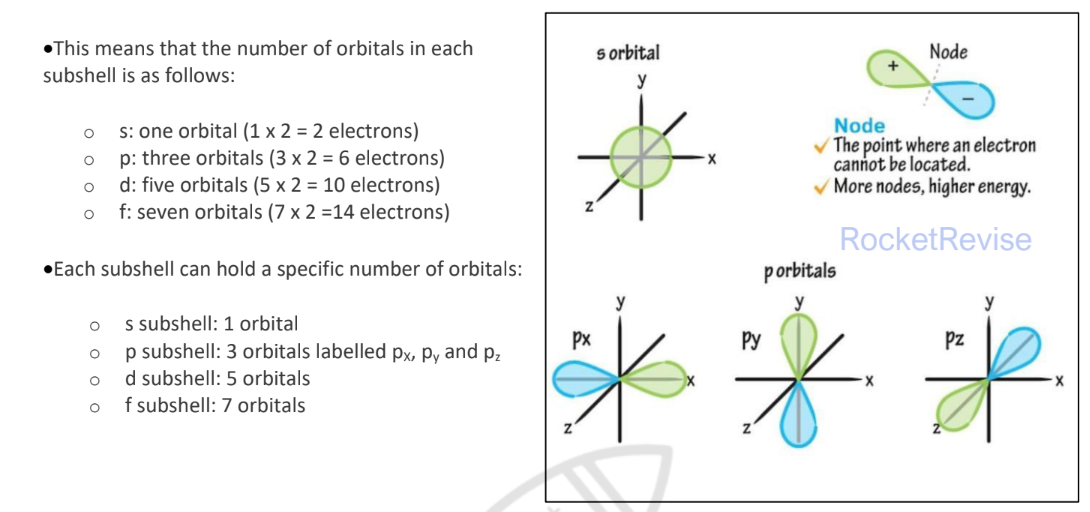
electron box notation
The electron configuration can also be represented using the electrons in boxes notation - each box represents an atomic orbital
The boxes are arranged in order of increasing energy from lowest to highest
The electrons are represented by opposite arrows to show the spin of the electrons
The box notation for titanium (22) is shown below:
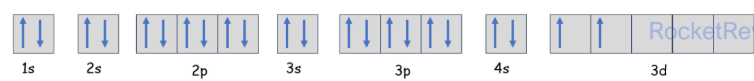
Free Radicals
A free radical is a species with one or more unpaired electron
the unpaired electron is shown as a dot
- E.g. a chlorine free radical has the electron configuration 1s2 2s2 2p6 3s2 3p5
- Two of the three p orbitals have paired electrons where as one of them has an unpaired electron
E.G. OF ELECTRONIC CONFIGURATION
Potassium has 19 electrons, so the full electronic configuration is:1s2 2s2 2p6 3s2 3p6 4s1
Calcium has 20 electrons, so the full electronic configuration is: 1s2 2s2 2p6 3s2 3p6 4s2
Abbreviated electron configuration of calcium is: (Ar) 4s2
The 4s orbital is lower in energy than the 3d subshell and is therefore filled first
Copper electronic configuration: 1s2 2s2 2p6 3s2 3p6 3d10 4s1
– because 3d10 is more stable than 3d9 (exception) – expected configuration is: 1s2 2s2 2p6 3s2 3p6 4s2 3d9
Chromium electronic configuration - 1s2 2s2 2p6 3s2 3p6 4s1 3d5
ionisation energy (IE)
The amount of energy required one mole of electrons from one mole of gaseous atoms of an element to form one mole of gaseous ion
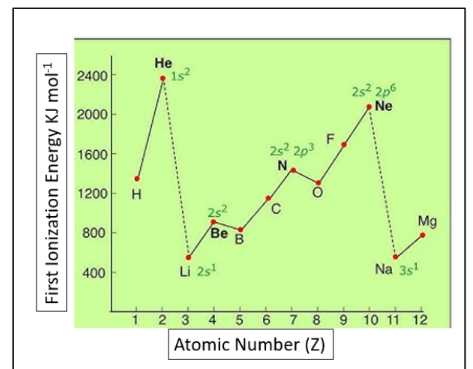
FACTORS AFFECTING THE SIZE OF IONIZATION ENERGY
Size of the nuclear charge: The nuclear charge increases with increasing atomic number - higher attractive forces between the nucleus and electrons - higher ionisation
distance of outer electrons from the nucleus: Electrons in shells that are further away from the nucleus are less attracted to the nucleus - the nuclear attraction is weaker - lower ionisation energy
Atomic radius: The lower the atomic radius, the closer the shell is to the nucleus - higher attractive forces - higher ionization energy (ionization energy increases across a period left to right)
Shielding effect of inner electrons: the more shells an atom has, the greater the shielding effect (inner shells prevents outer shell to feel full nuclear charge) - lower ionisation energy
Spin-pair repulsion: Electrons in the same atomic orbital in a subshell repel each other, this reduces energy needed to remove one of the electrons - lower ionisation energy
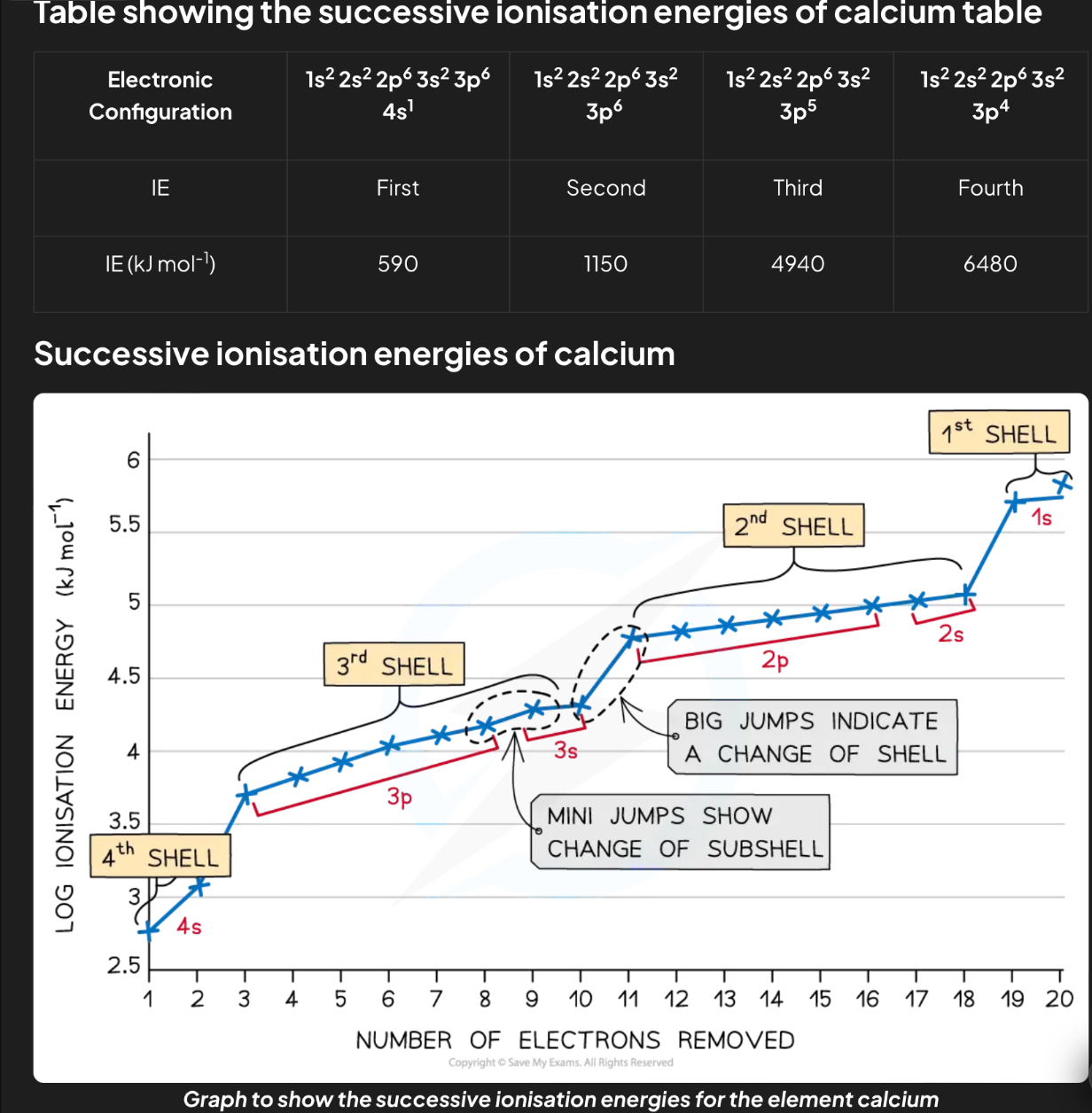
THE IONISSATION ENERGY INCREASES ACROSS A PERIOD DUE TO HIGHER NUCLEAR CHARGE AND SAME SHIELDING AND DECREASES DOWN A GROUP DUE TO GREATER ATOMIC RADII AND MORE SHIELDING
THERE WILL BE LARGE INCREASE IN IONISATION ENERGY AS IT MOVES TO THE NEXT SHELL
Successive ionisation energy
It is energy required to remove the first, second, third…. electron
As more electrons are removed
Shielding increases
the proton to electron ratio increases
attraction between nucleus and remaining electrons increases
relative atomic mass Ar
The relative atomic mass of an element is the mass of an atom compared to 1/12 of the mass of an atom of carbon-12
Unified atomic mass: one-twelfth of the mass of a carbon-12 isotope (1u = 1.66 × 10-27 kg)
The Ar has no units as its a ratio
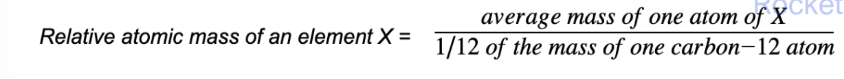
Relative isotopic mass
The mass of an atom of an isotope compared to one twelfth of the mass of an atom of carbon-12

Relative formula mass (Mr)

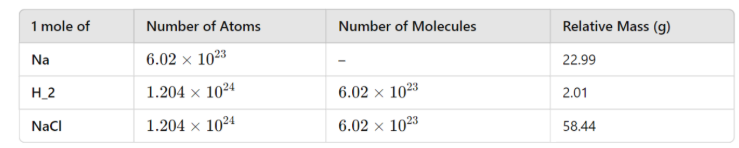
The Avogadro constant
The number of particles equivalent to the relative atomic mass of a substance
Avogadro constant applies to atoms, molecules, ions and electrons
The value of Na is 6.02 × 1023 g mol-1
Charges of ionic compounds
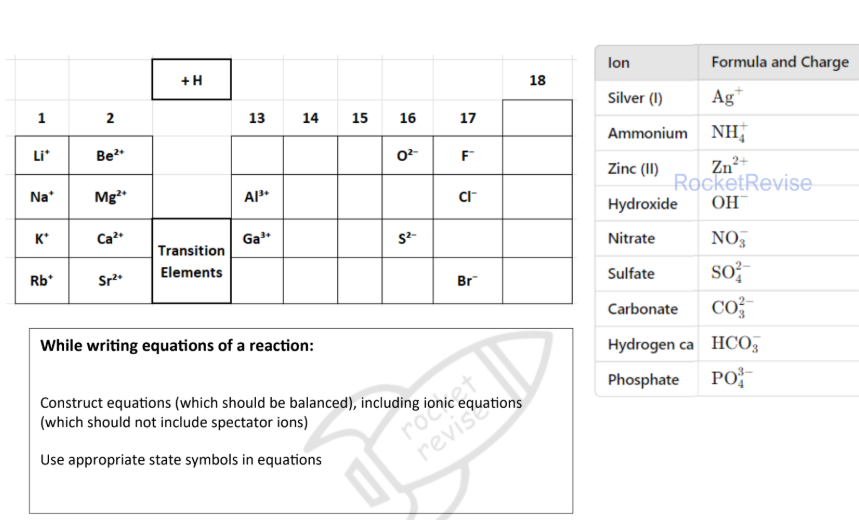
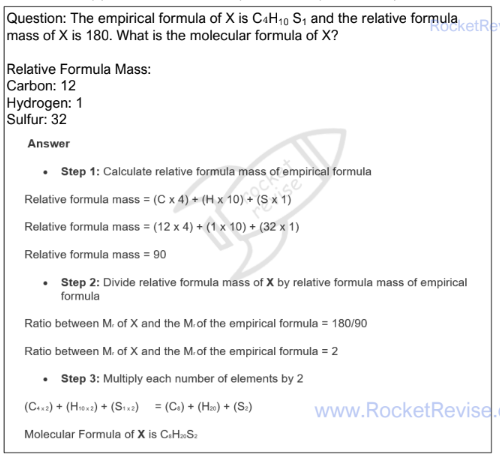
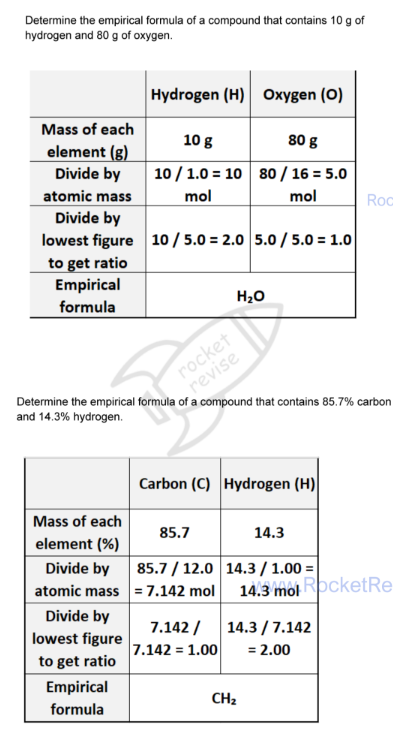
Empirical formula
The simplest whole number ratio of the elements present in one molecule or formula unit of the compound
Water of crystallisation
When some compounds can form crystals which have waters as part of their structure
A compound that contains water of crystallisation is a hydrated compound.
-The water of crystallisation is separated from the main formula by a dot when writing the chemical formula of hydrated compounds
o E.g. hydrated copper (II) sulfate is CuSO4∙5H2O
A compound that contains no water of crystallisation is a anhydrous compound
The conversion of anhydrous compounds to hydrated compounds is reversible by heating the hydrated salt
o Anhydrous to hydrated salt (by adding water): CuSO4 + 5H2O→ CuSO4∙5H2O
o Hydrated to anhydrous salt (by heating): CuSO4∙5H2O → CuSO4 + 5H2O
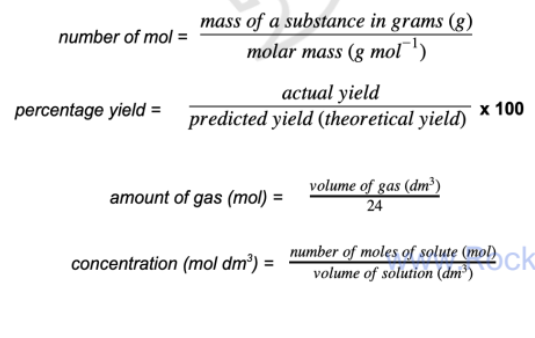
Important formulas
n = m/Mr
n = V/24
C = n/V
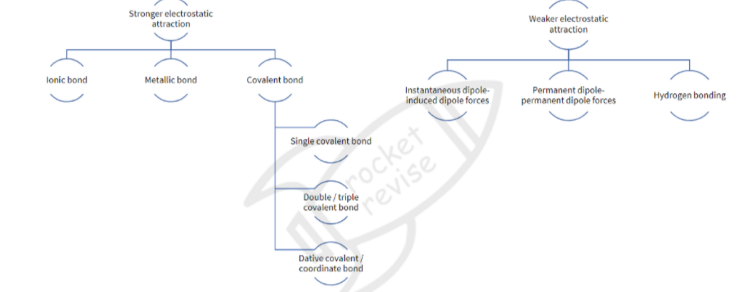
Bonding
Ionic Bond: The electrostatic attraction between oppositely charged ions (cations and anions)
Metallic bond: the electrostatic attraction between cations and delocalized electrons
Covalent bond: the electrostatic attraction between two nuclei of two atoms and a shared pair of electrons
Co-ordinate bonding/dative bonding: the sharing of a pair of electrons between two atoms where both the electrons in the bond come from the same atom
electronegativity: the power of an atom that is covalently bonded to another atom to attract the bonding pair of electrons to itself
Bond energy: the energy required to break one mole of that bond in a molecule that is in the gaseous state
Metals
high melting points
- a lot of energy is required to weaken the strong attractive forces between the metal ions and delocalized electronsStrength of metallic bonding increases with
- increasing positive charge on ions in metal lattice
- decreasing the size of metal ions in the lattice
- increasing the number of mobile electrons per atomelectrical conductivity
- Good conductor
- A electric current can flow across a metal because the delocalised electrons (mobile electrons) are free to moveheat conductivity
- good conductor
- thermal energy can be transferred by conduction - vibration caused by increased kinetic energy is passed on from one metal ion to the next
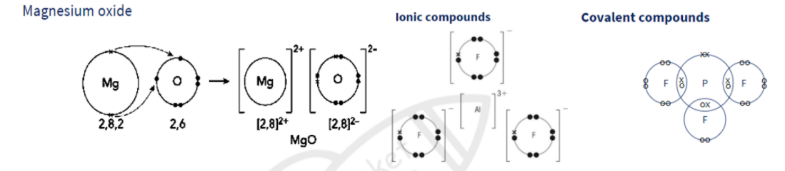
Ionic bonds
Ions are packed in regular arrangement called a lattice
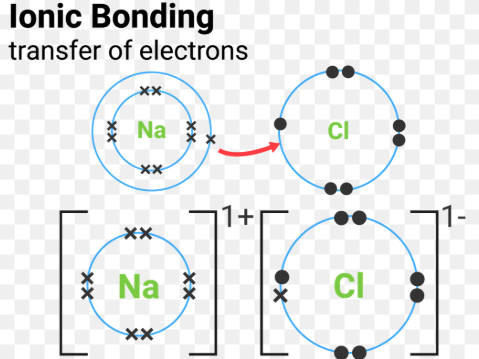
Covalent bond
bonding pair: a shared pair of electrons that are involved in covalent bonding
lone pair: pairs of electrons in the outer shell of an atom that are not involved bonding
single covalent bond: one shared pair of electrons bonding two atoms together
double covalent bond: two shared pairs of electrons bonding two atoms together
Triple covalent bond: three shared pairs of electrons bonding two atoms together
compounds with co-ordinate bond, incomplete octet or expanded octet
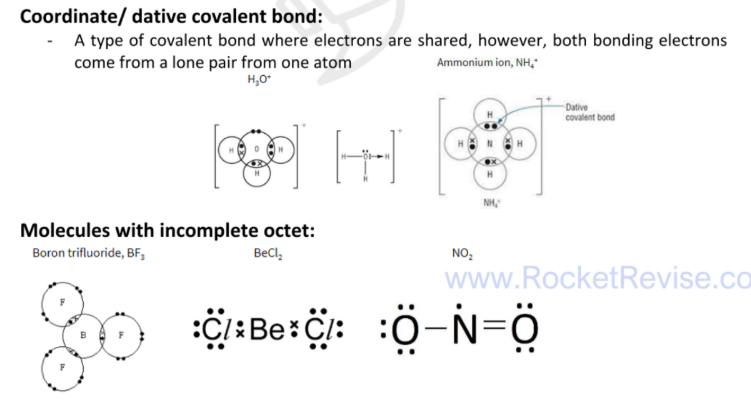
Coordinate/ dative covalent bond
a type of covalent bond where electrons are shared, however, both bonding electrons come from a lone pair from one atom
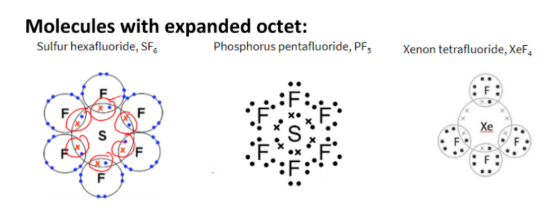
Dot-and-cross diagram
Dot-and-cross diagram shows:
the outer electron shells only
the charge of the ion is spread evenly, by using square brackets
the charge on each ion, written at the top right-hand corner
Molecular orbital and orbital hybridization
MOLECULAR ORBITAL
A combined orbital formed by two atomic orbitals overlapping to form a covalent bond
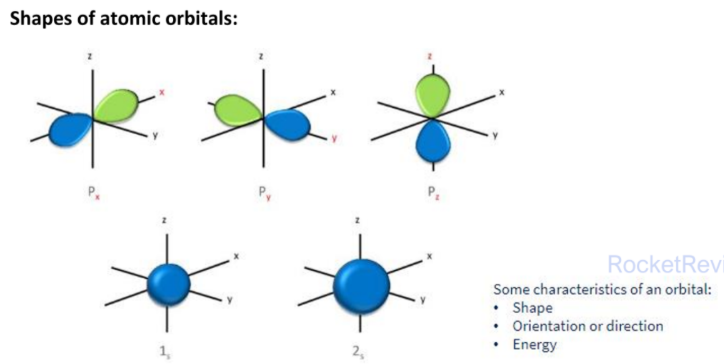
recall: an orbital is the region in space where electrons are most likely to be found
There are 2 ways to describe the type of orbital overlap in covalent bonds
- Sigma bond
- Pi bond
Pauli exclusion principle
Pauli exclusion principle requires that the maximum number of electrons in an orbital to be two
The two electrons in an orbital is of opposite spin
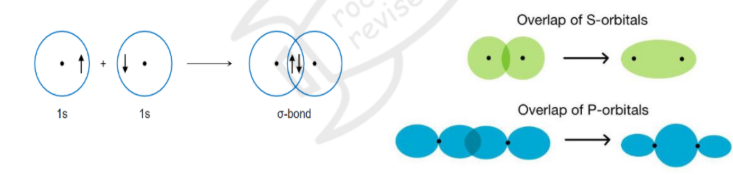
Sigma bond
sigma bonds are covalent bonds
it involves the end-to-end overlapping of an atomic orbital from one atom to another end of an atomic orbital from another atom
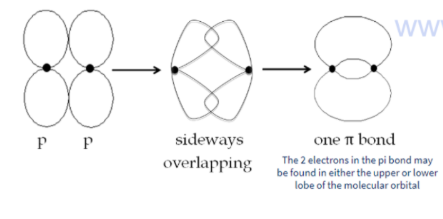
Pi bond
Pi bonds are also covalent bonds
it involves the side-by-side overlapping of two p orbitals
Pi bonds are weaker than sigma bonds
Pi bonds occur in double and triple bonds
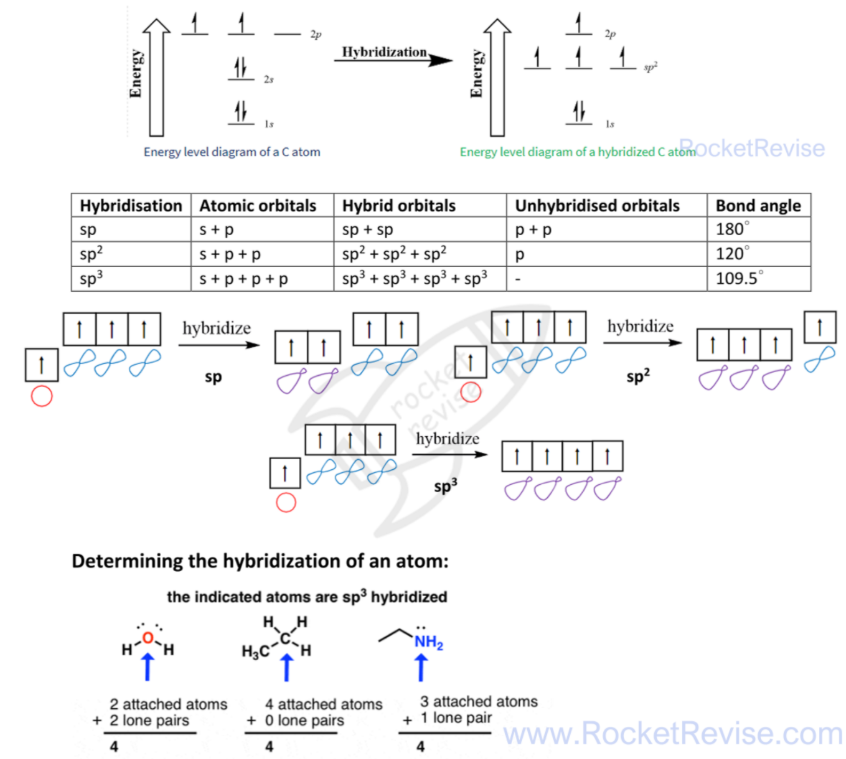
orbital hybridisation
each atomic orbital will participate in a covalent bond needs to be singly occupied( one electron in an orbital)
E.g. of C atom form 4 bonds
Determine sp, sp2,sp3 :
- sp don’t have pi bond
- sp2 have one pi bond
- sp3 have two pi bonds
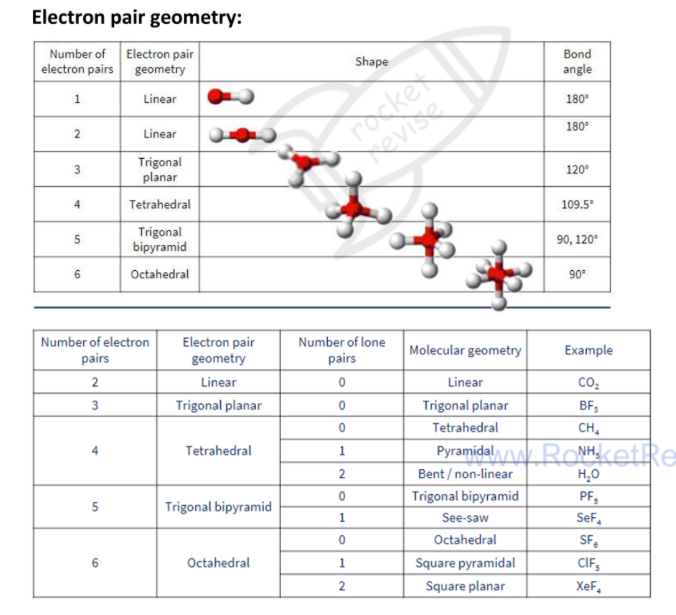
VESPR Theory
the shape of a molecule can be deduced using this theory, with the most stable shape being the one with the least repulsion between bonding and lone pairs of electrons
Bond energy and Bond strength
BOND ENERGY
bond energy of a bond is the energy required to break one mole of that bond in a molecule that is in the gaseous state
Pi bond is weaker than a sigma bond when comparing the same atoms
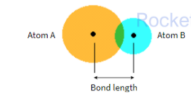
Bond length: is the internuclear distance between two atoms in a covalent bond
- Atoms with a larger radii produces molecules with greater bond length because the valence electrons are further from the nucleus
- A shorter bond length indicates a stronger bond due to greater electrostatic attraction between the two atoms
Electronegativity and bond polarity
ELECTRONEGATIVITY
The power of an atom (that is covalently bonded to another atom) to attract the bonding pair of electrons to itself
BOND POLARITY
The partial separation of charge when two different atoms are joined by a covalent bond, resulting in an unequal sharing for the bonding pair of electrons
Factors influencing electronegativity
NUCLEAR CHARGE
Atoms in the same period with greater nuclear charge are more likely to attract the bonding pair of electrons
ATOMIC RADIUS
Going down the group, atoms become less likely to attract the bonding pair of electrons
SHIELDING
the greater the shielding effect, the atom becomes less likely to attract the bonding electron
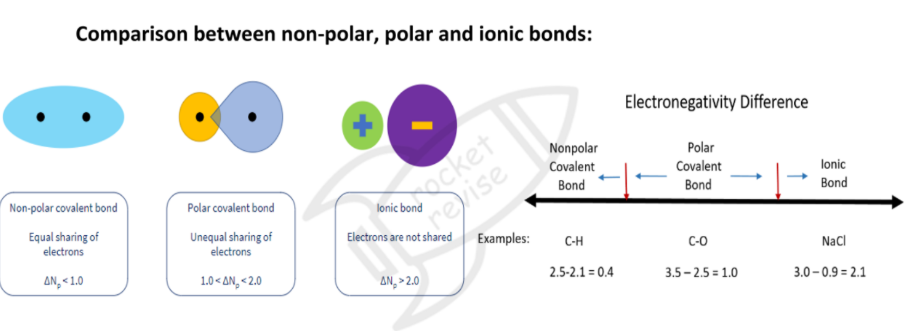
Pure and Polar Covalent Bond
Pure covalent bond: both atoms in a covalent bond have equal or similar electronegativities
- electron distribution is equal between the atom
- there is no separation of charge between atoms
- e.g. CL2, H2Polar covalent bonds: Two (bonded) atoms are partially charged - one atom is slightly positive and the other slightly negative
- Electron distribution is permanently unequal
- there is a separation of charge between atoms
- The bond has a dipole
- e.g. hydrogen chloride, HCL
Polarity
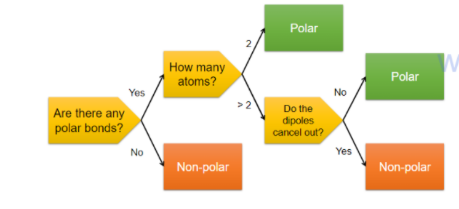
Polarity in molecules:
the overall dipole of a molecule depends on its shape and electron distribution
dipole moments are vector quantities
depending on the relative angles between the bonds the individual bond dipoles can either reinforce or cancel each other out
Dipole moment of molecules
The dipole moment of a molecule is the vector sum of the dipole moments of all the bonds
A molecule with net dipole moment (non-zero) is said to be polar
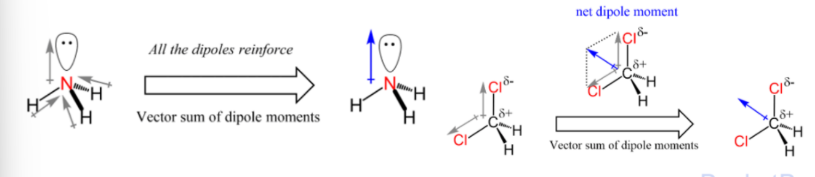
Bond polarity influences chemical reactivity
many chemicals reactions begin with a reagent attacks one of the electrically charged ends of polar molecule
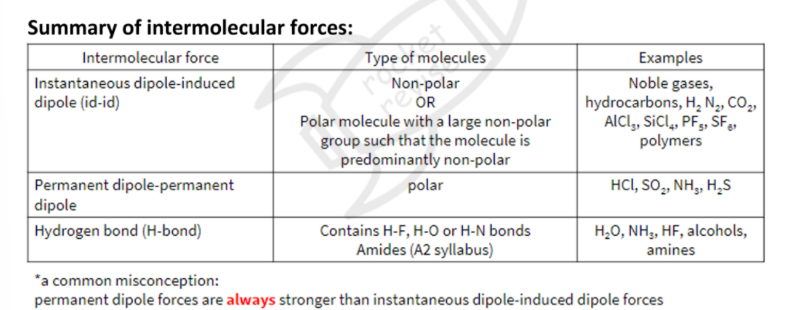
Intermolecular forces (IMFs)
Exist between discrete covalent molecules
IMFs are due to the formation of temporary and/ or permanent dipoles
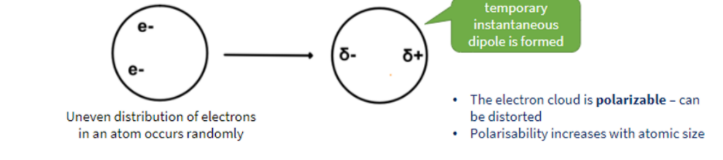
INSTANTANEOUS DIPOLE-INDUCED DIPOLE FORCES
Attractive intermolecular forces caused by temporary instantaneous dipoles in molecules
- observed in all (polar and non-polar) molecules
- strength depends on number of electrons and size
PERMANENT DIPOLE-PERMANENR DIPOLE FORCES
Attractive intermolecular forces caused by permanent dipoles in molecules
-observed in polar molecules
- Strength depends on charge (electronegativity difference)
Hydrogen bonding
Observed for H bonded to F,O or N
Strongest amongst the intermolecular forces
hydrides of period 2 elements have unusually high boiling points
EFFECTS OF HYDROGEN BONDING
H2O has 2 H bonds per molecule, hence:
- higher boiling point than NH3 and HF
- high viscosity (more fluid) compared to molecules of similar Mr
- ice is less dense than water:
H2O arranged in a rigid lattice in ice
less densely packed compared to water
OTHER EFFECTS
Surface tension
- attractive forces of molecules at the surface of a liquid that allows it to resist an external force and reduce its surface areaSolubility
- polar compounds dissolve in polar solvent
- Non polar compounds do not dissolve in non-polar solvents
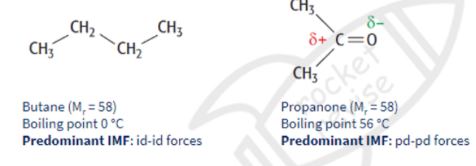
Permanent dipole-Permanent dipole forces
attractive forces between the sigma 8+ charge on one molecule and the 8- charge on another molecule - both molecules have permanent dipoles
For small molecules with the same number of electrons, pd-pd forces are often stronger than id-id forces
Melting points of halogens vs hydrogen halides
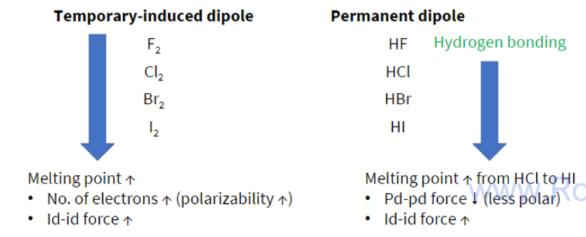
id-id and pd-pd
Kinetic theory of gases
gas molecules move rapidly and randomly
all collisions between particles are elastic
-No kinetic energy is lost due to collisionsdistance between gas molecules is greater than diameter of molecules
No forces of attraction / repulsion between molecules
The temperature of the gas is related to the average kinetic energy of the molecules
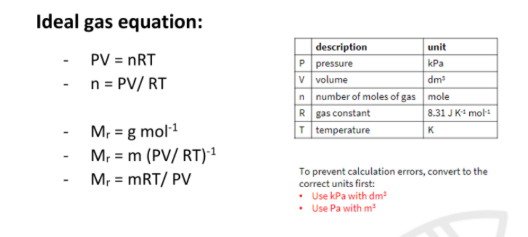
Ideal gas
PV = nRT
IDEAL GAS
Volume of molecules is negligible
no forces of attraction/repulsion
REAL GAS
volume of molecules is not negligible
forces of attraction/repulsion between molecules
Giant ionic lattice
A 3d arrangement of alternative positive and negative ions
ions held by strong electrostatic forces
PROPERTIES
Hard - strong electrostatic attraction between ions
brittle
high melting and boiling points (high charge density)
conducts electricity when molten or aqueous (mobile ions)
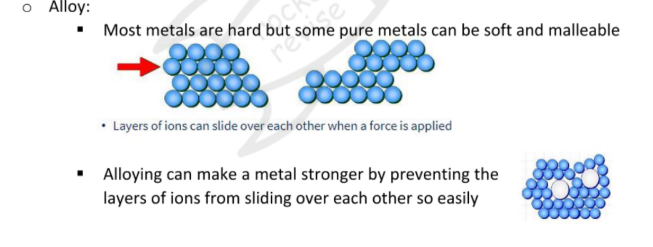
Giant metallic lattice
A 3d arrangement of positively charged ions surrounded by a sea of delocalised electrons
PROPERTIES
Malleable and ductile
- metallic bonds are easily broken and reformed
- delocalized electrons continue to hold the ions in the latticeHigh tensile strengh
- due to strong attractive forces between metal ions and delocalized electrons
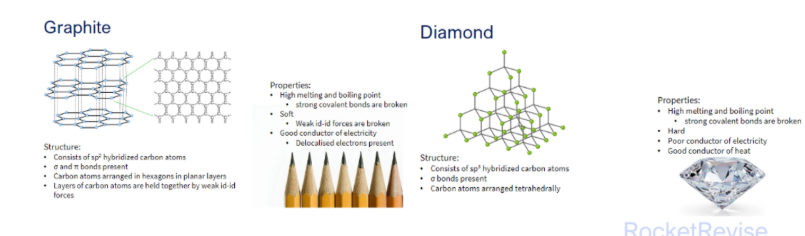
Giant covalent/molecular lattice
3d network of covalent bonds throughout the whole structure
Properties:
Diamond and SiO22
- high melting point and boiling point - strong covalent bonds are broken
- Hardness
- Does not conduct electricity
Simple molecular/simple covalent
a 3d arrangement of covalent molecules held together by weak intermolecular forces
properties:
- low melting / boiling point
Activation energy
The minimum amount of energy required for reactant molecules to have successful collision and start the reaction
Standard enthalpy changes
STANDARD ENTHALPY CHANGE OF REACTION, H0r
Is the enthalpy change when the reactants shown in stoichiometric equation react to form products, with all species in their standard states
can be both exothermic and endothermic
STANDARD ENTHALPY CHANGE OF FORMATION, H0f
the enthalpy of one mole of compound is formed from its elements in their standard states
can be both endothermic and exothermic
STANDARD ENTHALPY CHANGE OF COMBUSTION, H0c
the enthalpy change when one mole of a substance, in its standard state, is burnt in excess oxygen
exothermic
STANDARD ENTHALPY CHANGE OF NEUTRALISATION, H0neut
the enthalpy change when one mole of water is formed by reacting an alkali and base
it is exothermic
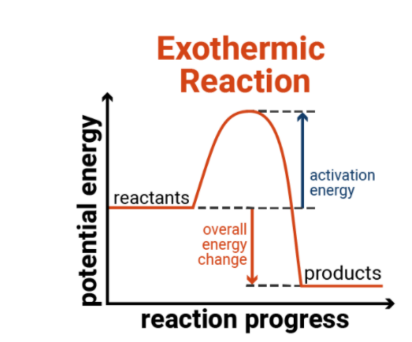
Exothermic reaction
Wen the products have less energy than the reactants
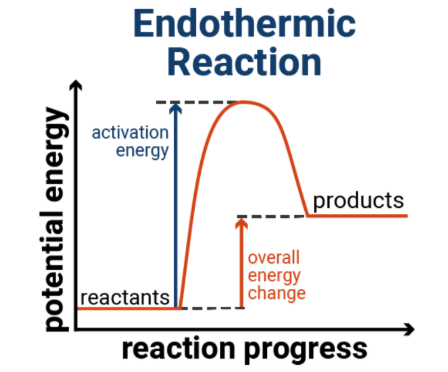
Endothermic reaction
When the products have more energy than the reactants
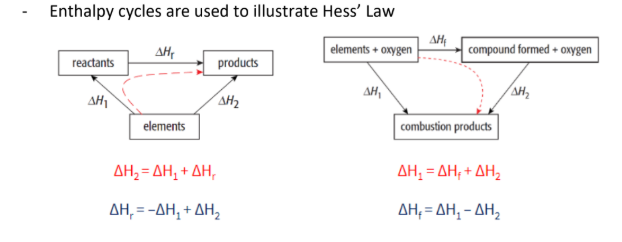
enthalpy cycles
HESS’S LAW
The total enthalpy change in a chemical reaction is independent of the route by which the chemic reaction takes place (as long as the initial and final condition are the same)
Oxidation
gain of oxygen
loss of hydrogen
loss of electrons
increase in oxidation number
Reduction
gain of hydrogen
loss of oxygen
gain of electrons
decrease in oxidation number
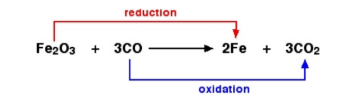
Redox reaction
oxidation and reduction always occur simultaneously in a chemical reaction
OXIDISING AGENT
A substance which oxidises another substance and itself is reduced
REDUCING AGENT
A substance which reduces another substance and itself is oxidised
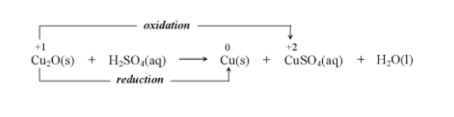
Disproportionation
A redox reaction in which both oxidation and reduction occurs on the same atom. The atom is simultaneously oxidised and reduced
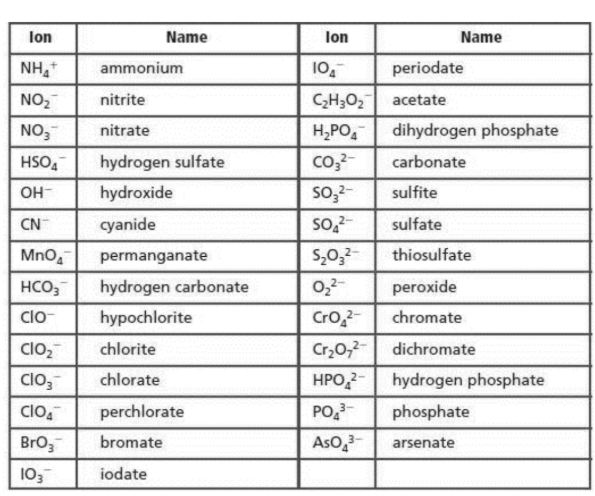
Names of compounds
Reversible reaction
A reaction in which products can be changed back to reactants by reversing the condition

equilibrium reaction
it is dynamic, the forward and revers reaction occur at the same rate
the concentration of the reactants and products remain constant
It requires a closed system - no loss of reactants or product
FACTORS AFFECTING POSITION OF EQUILIBRIUM
concentration
pressure
temperature
catalyst
Le Chatelier’s principle
If a change is made to a system in dynamic equilibrium, the position of the equilibrium moves to minimize this change
concentration on dynamic equilibrium
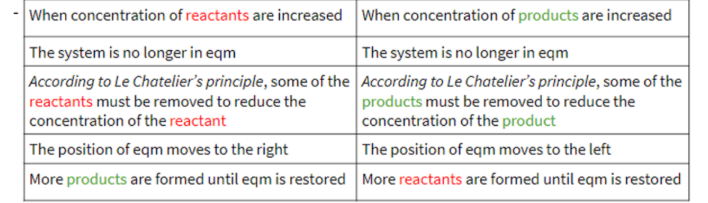
pressure on dynamic equilibrium
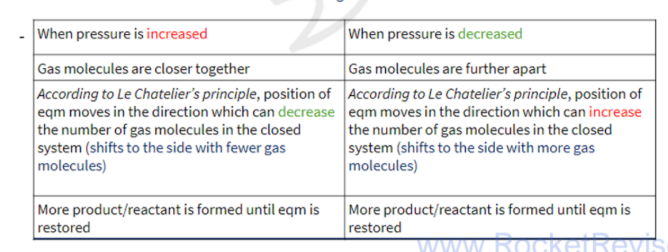
temperature on dynamic equilibrium
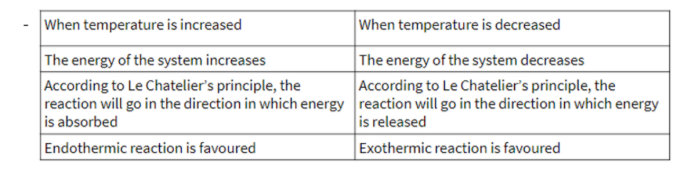
catalyst on dynamic equilibrium
catalyst helps to speed up a reaction by providing a path with lower activation energy
catalyst help to speed up both the forward and backward reaction, therefore does not affect the position of equilibrium