4. Rate-Reaction time graphs + catalysts
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
7 Terms
Write down the corresponding graphs to all the orders
in textbook page 304
How do you determine if an unclear graph is linear or curved?
log both sides and make it into the form y=mx +c
plot the logged values on the graph.
What does Quenching mean?
Ways to Quench your reaction mixture - 3
Stopping the reaction occurring in the reaction mixture
place in ice, dilute with a lot of distilled water, neutralise the reactant we are not measuring.
Concentrations vs time graphs:
How to determine the concentration of a reactant at a particular time
Set up the reaction mixture and start the reaction, and at the same time start a timer.
At regular time intervals, remove a sample from the reaction mixture to measure the leftover reactant’s concentration.
Quench the reaction to stop the concentration from changing and then measure the concentration.
Analyse the concentration of left over reactant → e.g. titration
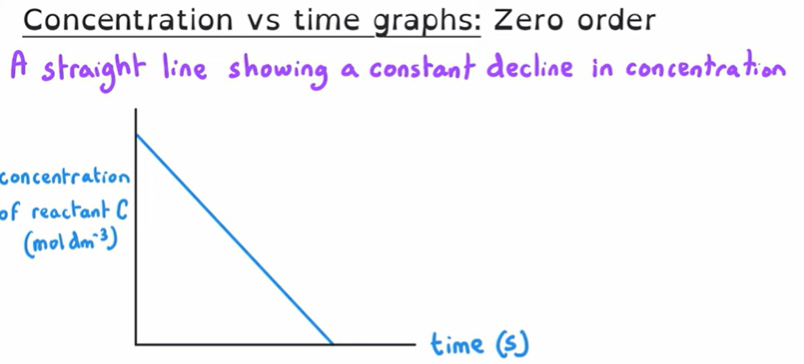
What is the order of this graph?
Zero order
Because even though we are seeing a decrease in concentration, the gradient is unchanged.
Definition of a catalyst - 4 main points
A substance that speeds up the rate of reaction without it being used up by providing an alternative reaction pathway with a lower activation energy, increasing the frequency of successful collisions between reactant particles.
What type of equations are catalysts included and no included in
Catalysts appear in rate equations
Catalysts don’t appear in the chemical equation.