Chemistry unit test 3
5.0(1)
Card Sorting
1/29
Last updated 8:48 AM on 11/10/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
1
New cards
The s-block
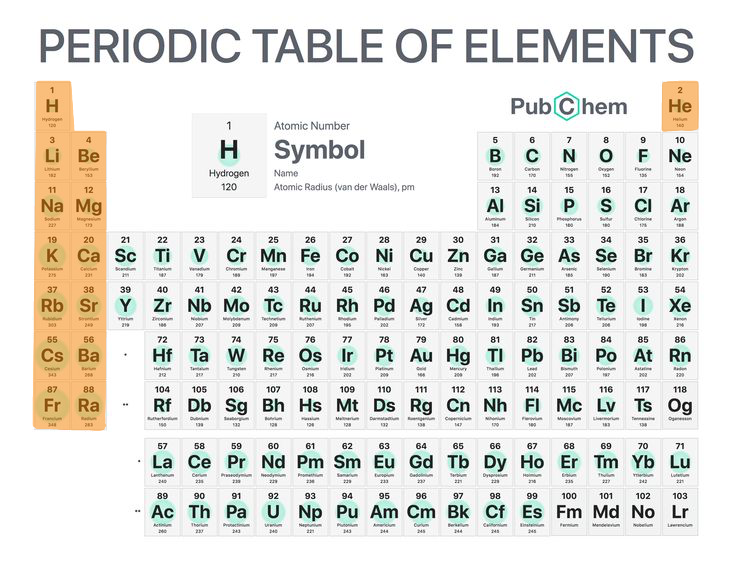
2
New cards
The p-block
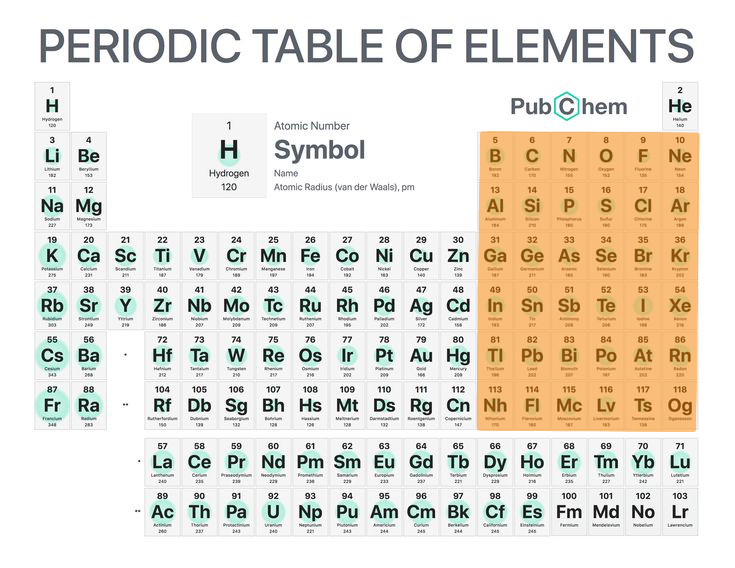
3
New cards
Metals
Kind of mineral substances.
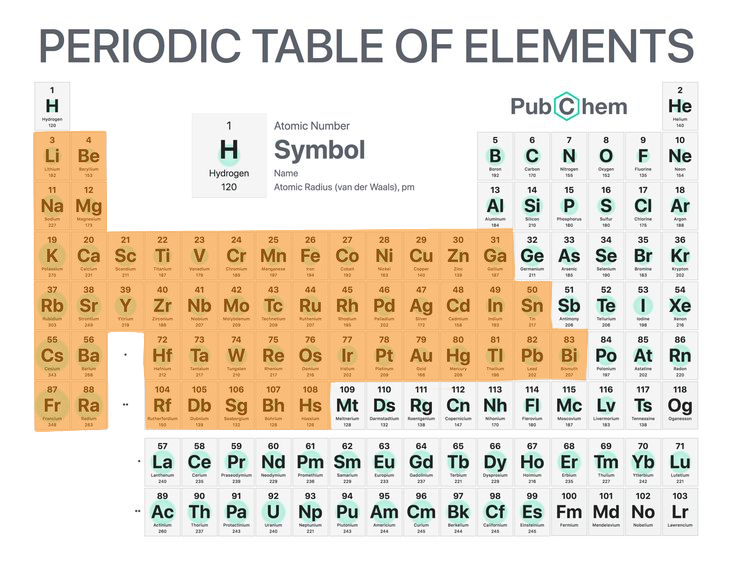
4
New cards
Non-metals
Chemical elements that are not metals.
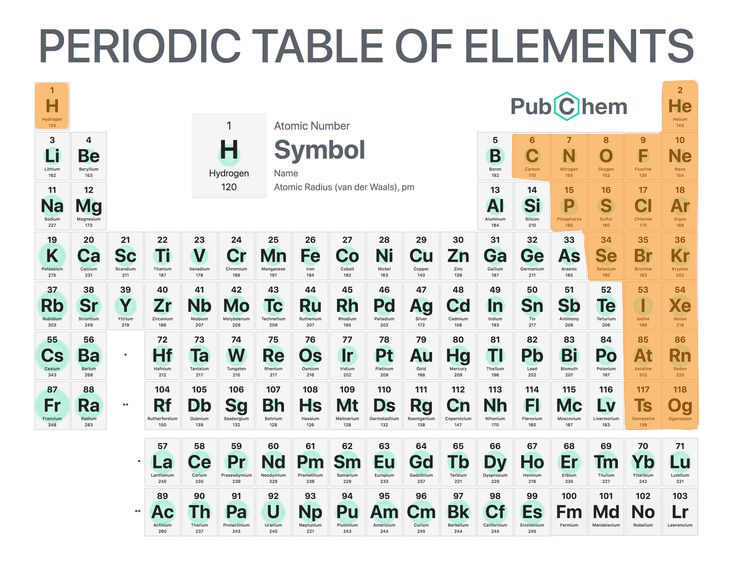
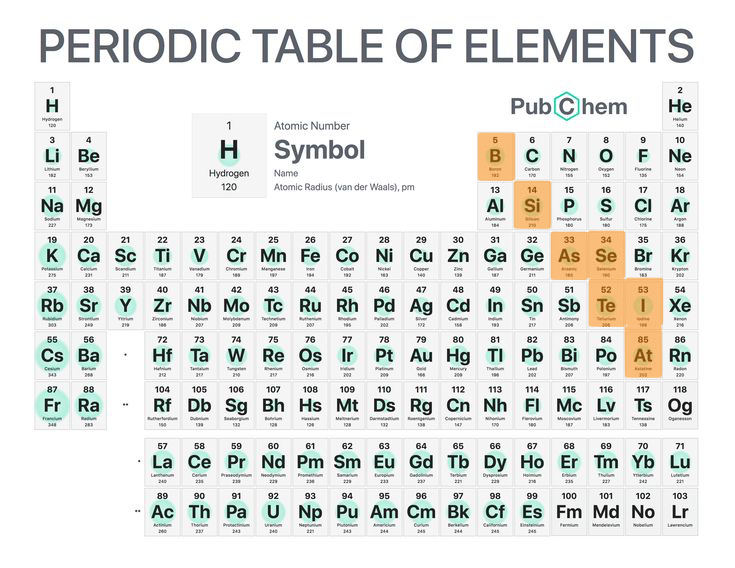
5
New cards
Metalloids
Chemicals elements with some of the properties of metals/non-metals.
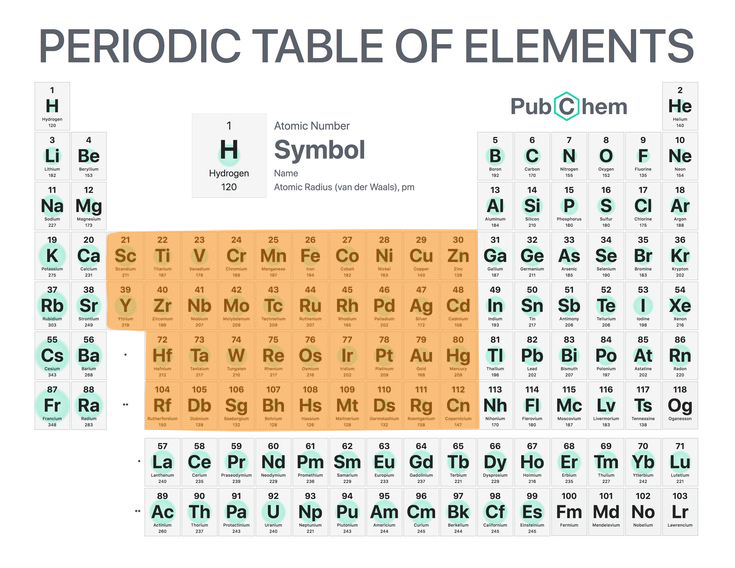
6
New cards
Transition metals
Normal metals (group 3 -> group 12).
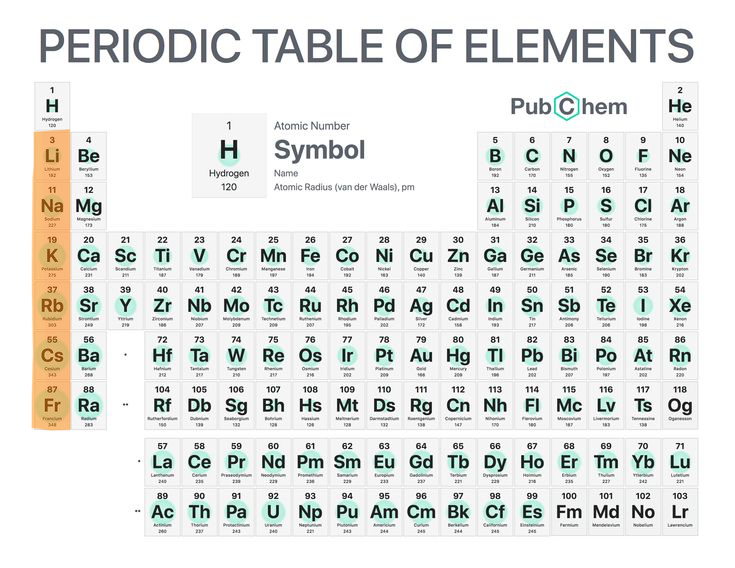
7
New cards
Alkali metals
They are very reactive (group 1 except hydrogen).
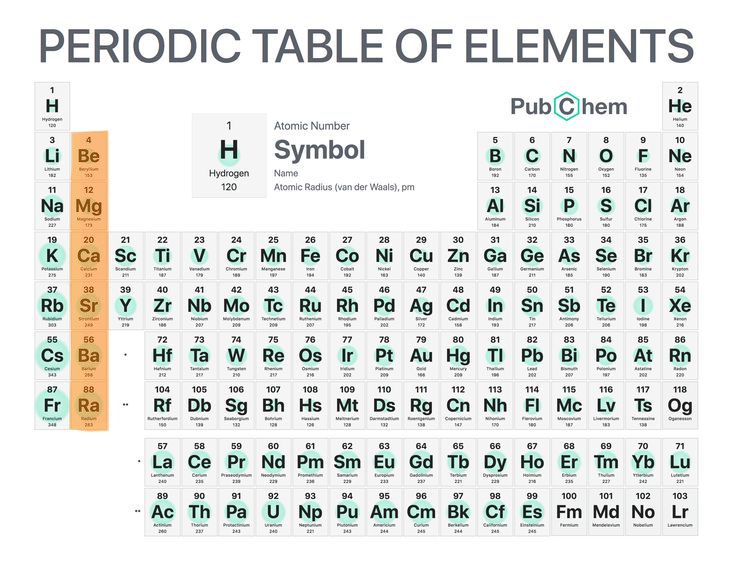
8
New cards
Alkaline earth metals
Reactive but not so much as the alkali metals (group 2).
9
New cards
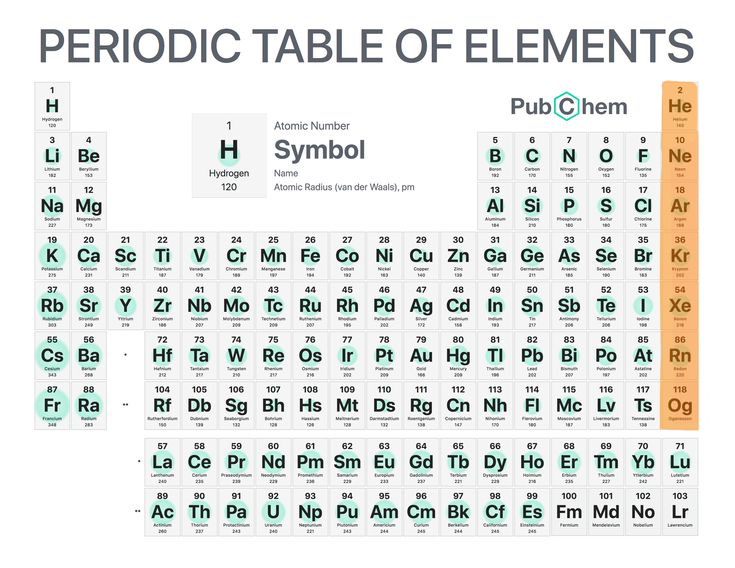
Noble gasses
Non reactive non-metals (group 18).
10
New cards
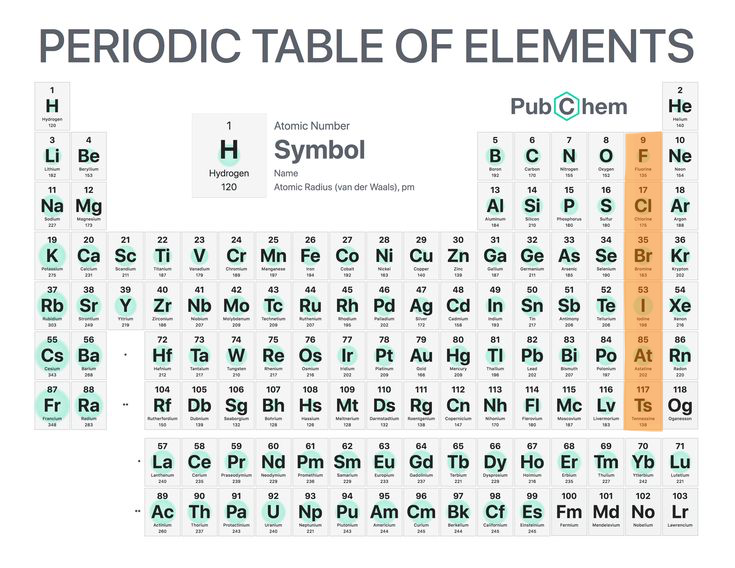
Halogens
Very reactive non-metals (group 17).
11
New cards
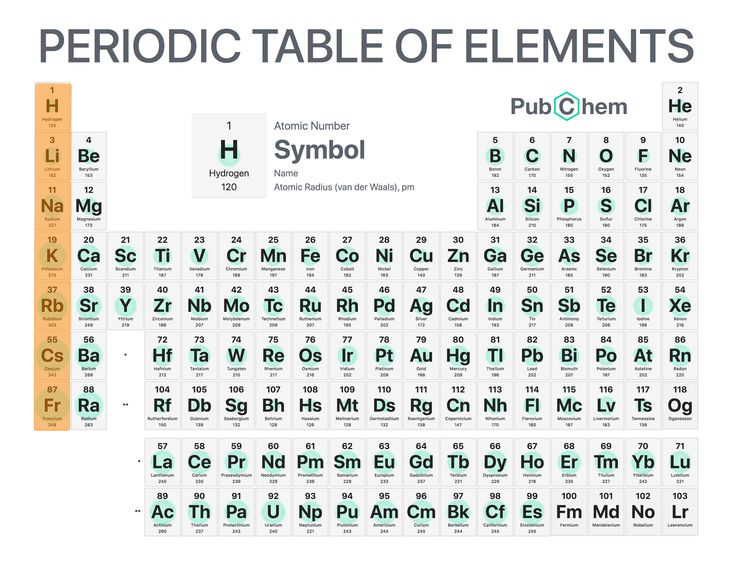
Group
The vertical row of the periodic table and has properties of elements in the same ______ are similar.
12
New cards
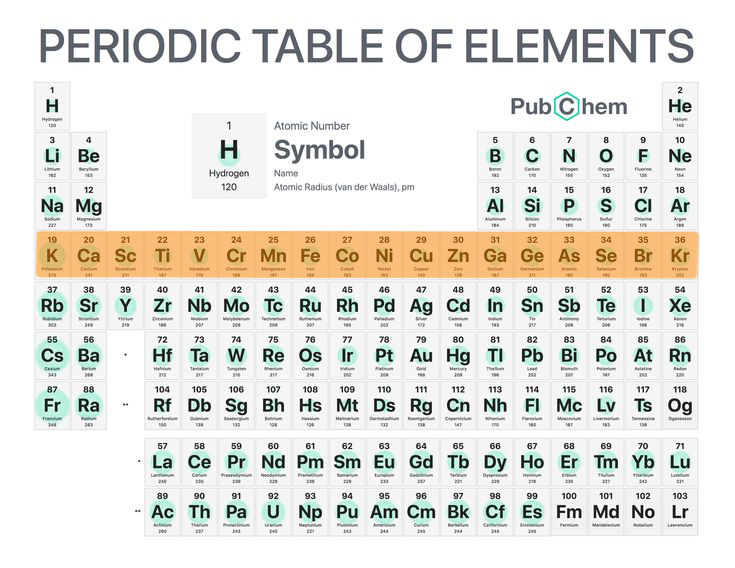
Period
The horizontal row of the periodic table and has properties of elements change accross a _____.
13
New cards
Periodic law
When elements are arranged in order of increasing atomic number, there are repeating patterns in their physical/chemical properties
14
New cards
Conductors
Materials that let heat and/or electricity past through.
15
New cards
Malleable
You can change the shape of a material without breaking it.
16
New cards
Ductile
Can be bent or stretched easily
17
New cards
Brittle
Delicate and easily broken.
18
New cards
Dull
Not shiny.
19
New cards
great; malleable; ductile; shiny; solids; high
Metals are ____ conductors of heat and electricity. They are also m__, d___ and have a ____ surface. Because metals are ___ at room temperature so they have ___ melting points.
20
New cards
gasses; brittle; dull; poor
Most nonmetals are ___ at room temperature, but some are b___ and d___ solids. They are ____ conductors of heat and electricity.
21
New cards
similar; similar
Metalloids are _____ to metals in someways, and _____ to nonmetals in other ways.
22
New cards
1869
The periodic table was created in ____
23
New cards
increasing; atomic number; groups; periods
In the modern periodic table, elements are arranged by the _____ of _____. They are laid out in g___ and p___ that highlight patterns in their chemical/physical properties.
24
New cards
Physical properties
The characteristics you can observe/measure without changing what the substance is made of.
25
New cards
Chemical properties
How a substance forms compounds with other substances (you cannot observe/measure this without changing what the substance is made of)
26
New cards
Cations
Types of ions are usually formed by metals and have positive charge.
27
New cards
Anions
Types of ions are usually formed by nonmetals and have negative charge.
28
New cards
Ions
An atom that has a positive/negative charge because it has gained/lost electrons. Atoms achiece a stable noble gas electron configuration by either losing all their valence electrons/gaining enough electrons to fill up their outer energy level.
29
New cards
Noble gas electron configuration
The elemental symbol of the last noble gas prior to that atom, followed by the configuration of the remaining electrons.
30
New cards
Ionization energy
The energy needed to remove an electron from an atom (measured in k/mol).