quantum numbers
1/8
Earn XP
Description and Tags
20/7/25 + half of 25/5/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
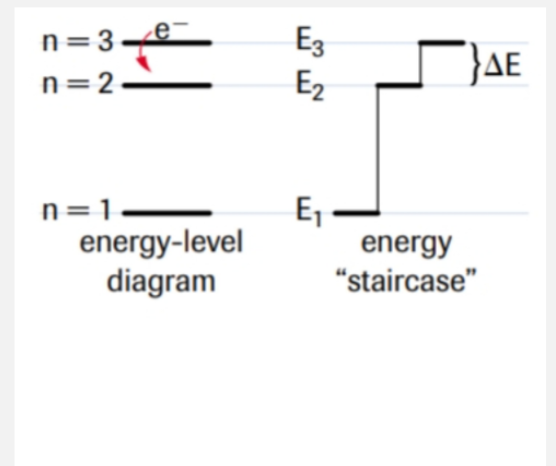
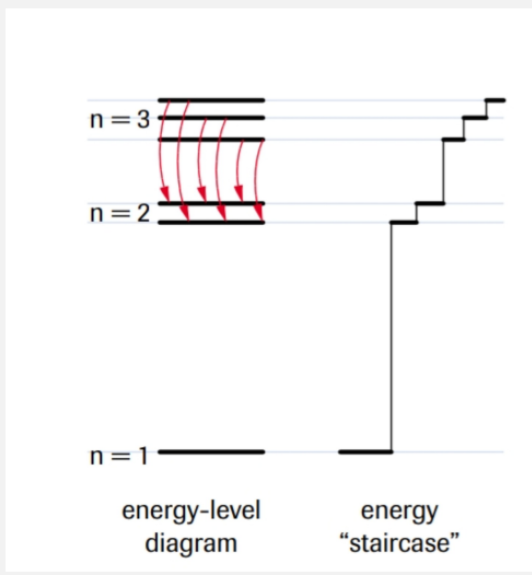
principal quantum number
energy level of electron
n=1, 2, 3, 4…
secondary quantum number
subshell/orbital shape
l = n - 1
if n = 3, l = 0, 1, 2
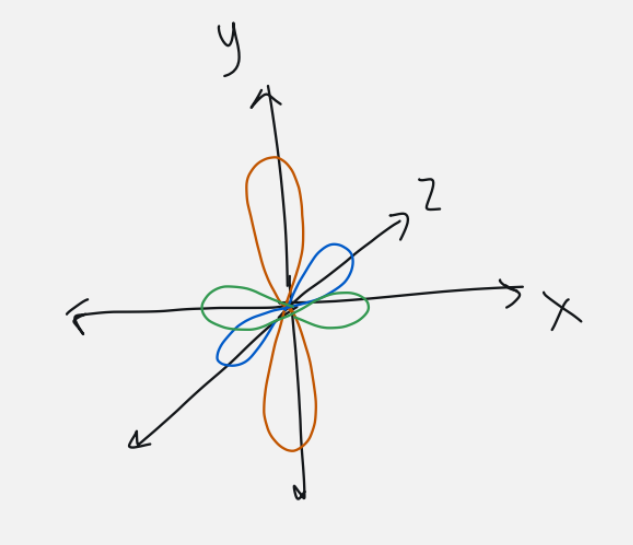
magnetic quantum number
orbital/subshell orientation (shape)
ml = -l … 0… +l
if l = 1, ml = -1, 0, 1
if l = 3, ml = -3, -2, -1, 0, 1, 2, 3
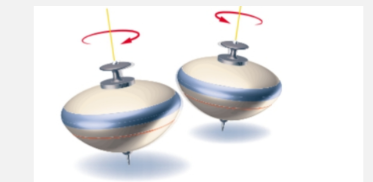
magnetic spin number
spin of electron
ms = +1/2 (clockwise spin), -1/2 (counterclockwise spin)
what are the purposes of quantum #s?
estimate location of electron
s - orbital
l = 0
1 orbital: ml = 0
2 electrons
spherical
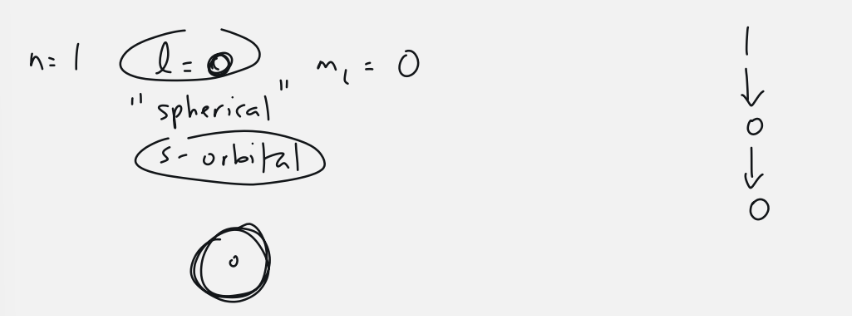
p-orbital
l = 1
3 orbitals: ml = -1, 0, 1
6 electrons
dumbell
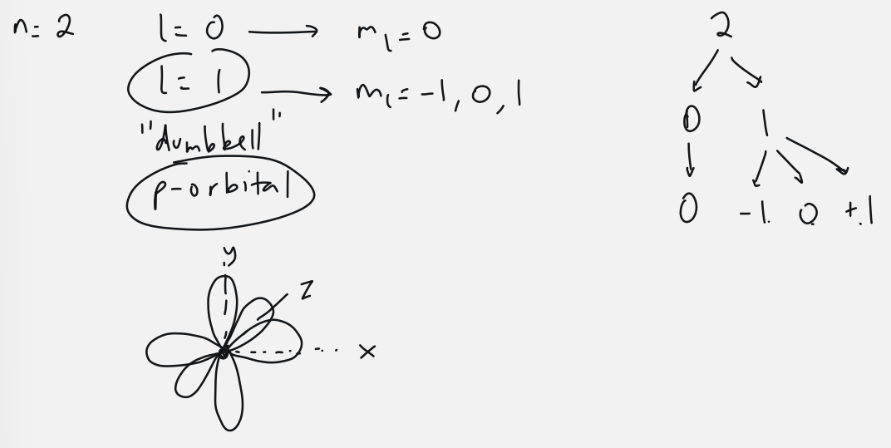
d-orbitals
l=2
5 orbitals: ml = -2, -1, 0, 1, 2
10 electrons
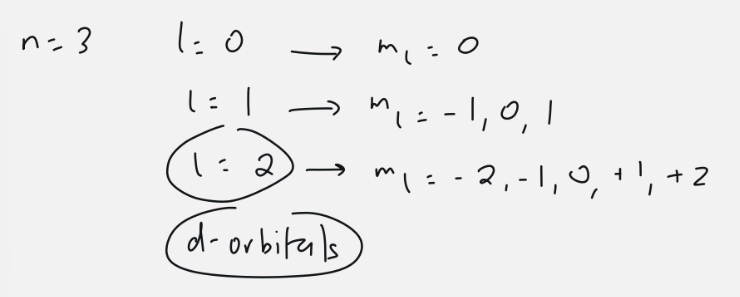
f-orbitals
l = 3
7 orbitals: ml = -3, -2, -1, 0, 1, 2, 3
14 electrons