lec 23 - gene therapy II (guo)
1/50
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
51 Terms
delivering genes to patient cells overview
to deliver a gene to patient cells we can use: 1) viruses or 2) non-viral methods
when using viruses, first we haev to find a way to get the desired gene into the virus
to get the desired gene into viruses, we use specifically engineered eukaryotic cells grow in the lab. these cells are engineered with genes (called gene sequences) that express viral proteins → b/c they supply viral proteins, they are called packaging cells
scientists grow eukaryotic cells and then modify these cells to give them genes that make viral proteins (gag/pol, env; tools to make virus structure) → these cells now produce the viral proteins needed to build the virus but they do NOT have the full viral genome → b/c these cells package the viral proteins to help assemble the virus they are called packaging cells
if we transfer the desired gene linked to the correct packaging sequences (called cis sequences) into the packaging cells, the packaging cells will start making viruses carrying the desired gene. such viruses that carry the desired gene are called vector virus
finally the vector viruses that carry the desired gene can be collected and used to infect target cells from the patient; upon infection, the vector virus will deliver the desired gene to the target cell
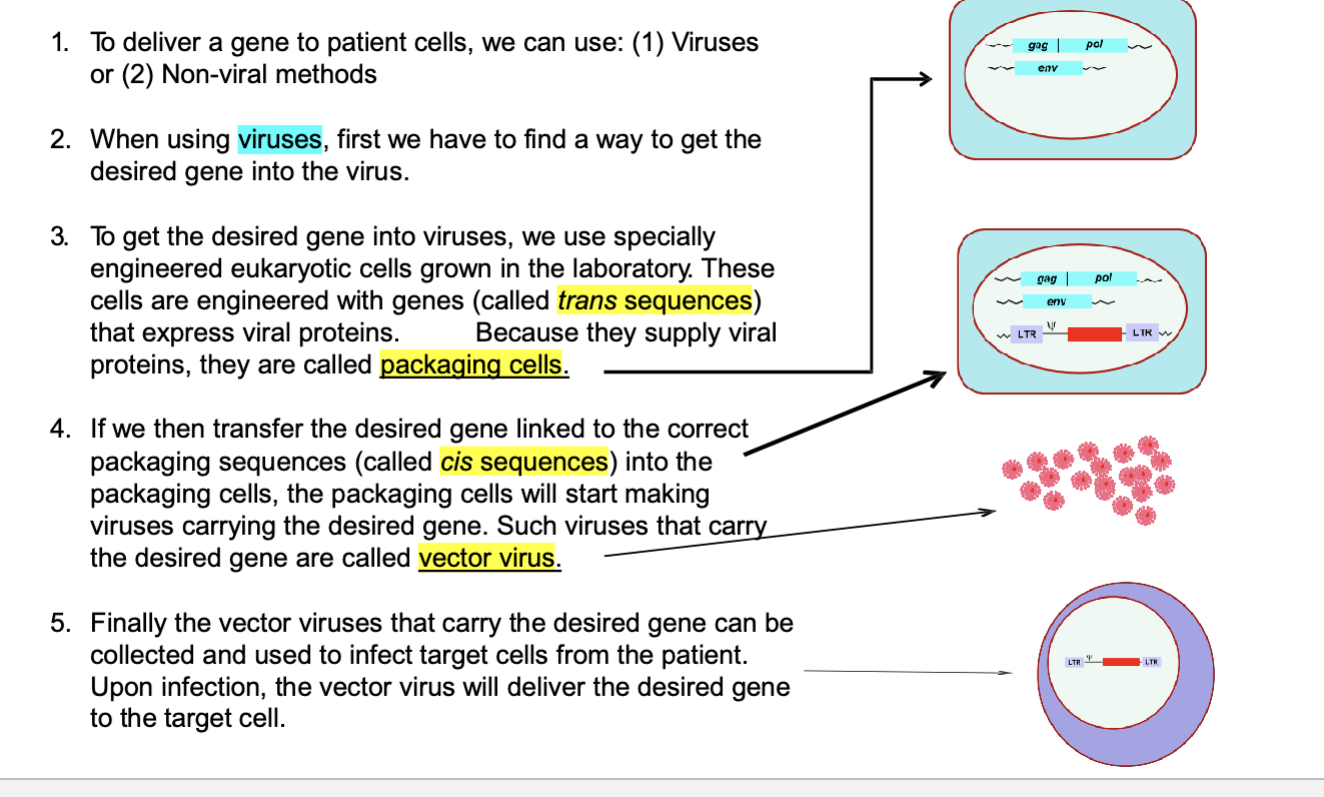
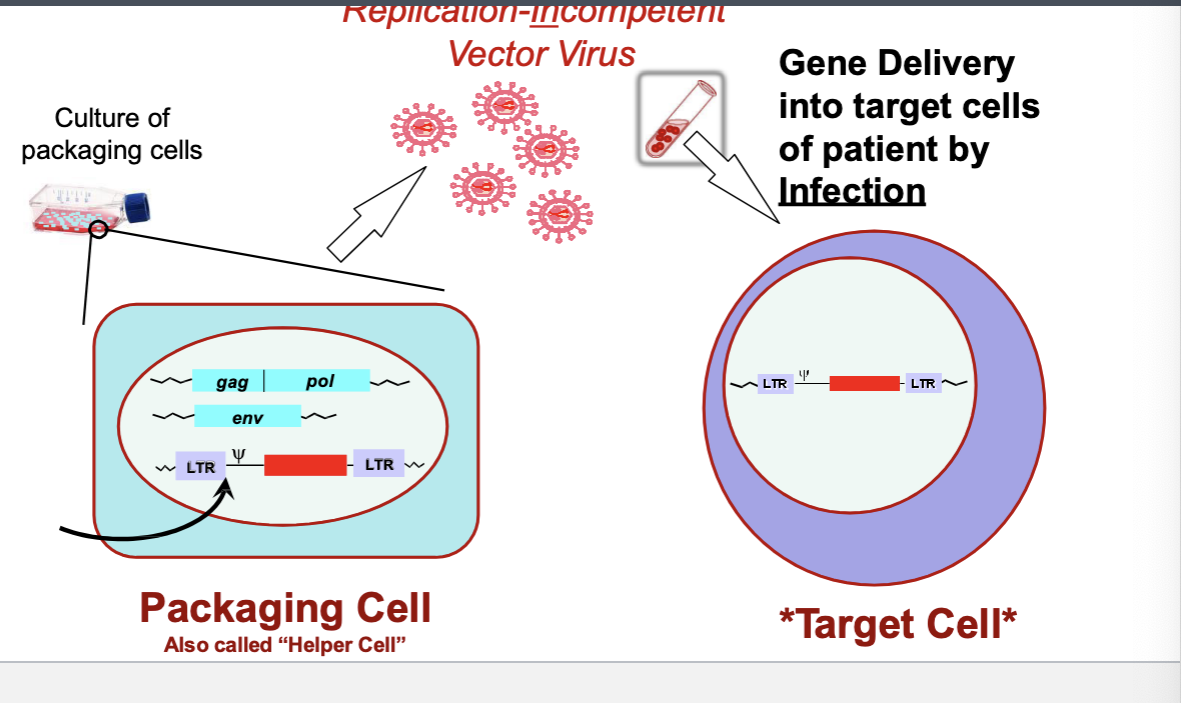
vector virus production and infection
packaging cell with trans/cis parts → replication-incompetent vector virus → gene delivery into target cells of patients by infection
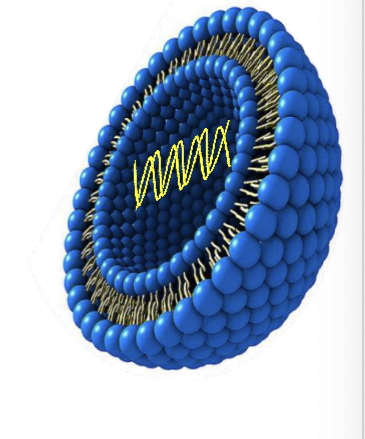
non-viral gene delivery systems
liposomes
spherical vesicles composed of synthetic lipid bilayers that miimc the structure of biological membranes
cationic (pos charge) lipids used for transferring DNA (neg charge) into cells
the transgene is packaged in vitro with the liposomes and used directly for delivering the DNA to a suitable target tissue in vivo
lipid coating protects the DNA in vivo and binds to cells
packaged DNA enters into cells by direct fusion
efficiency of transfer is relatively low and the introduced foreign gene does NOT integrate into hosts genome
high liposome and DNA concentrations required but both are relatively easy and cheap to manufacture in large amounts
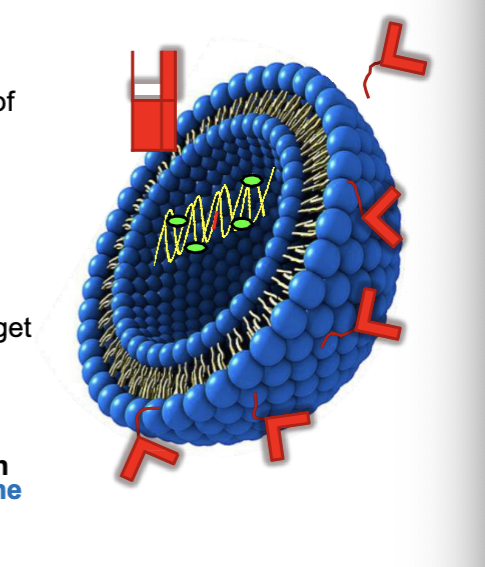
improved targeting of liposomes
PEGylated liposomes
PEG (improved PK/PD) improves the half life of liposomes
transferrin-conjugated liposome
transferrin can binds transferrin receptors found on human cells
antibody modified liposomes
antibody coating for specificity
adding a sendai (HVJ) viral proteins
allows DNA to escape the endosome intact by disrupting endosomal membrane
DNA binding proteins (HMG-1 high mobility group 1 protein)
to target foreign DNA into nucleus
nuclear localizing sequence (NLS) within DNA
as a wide range of DNA encoding both small or large proteins can be accommodated → liposomes offer very versatile option for gene delivery
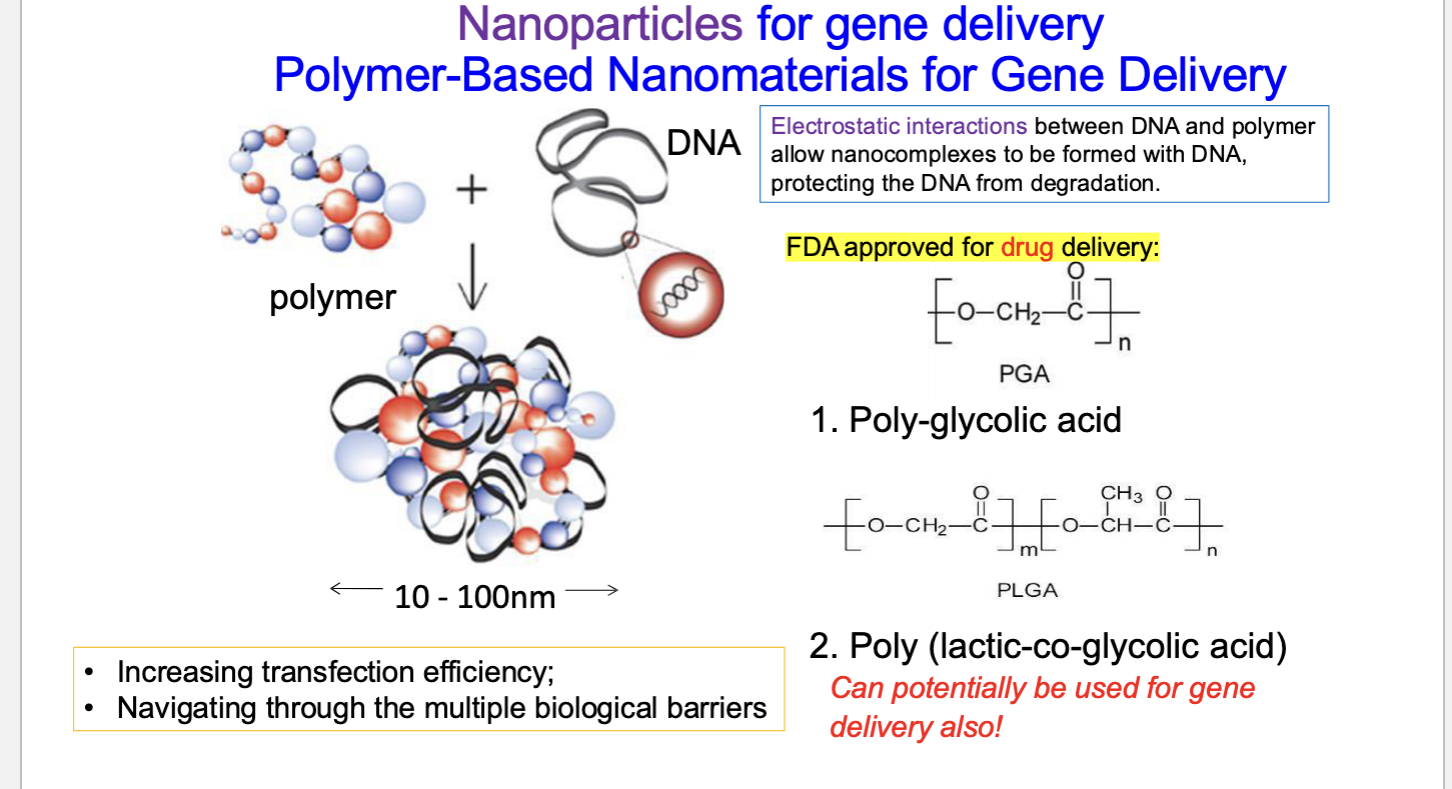
nanoparticles for gene delivery: polymer-based nanomaterial for gene delivery
electrostatic interactions between DNA + polymer allow nanocomplexes to be formed with DNA → protects DNA from degradation
PGA (poly-glycolic acid) = FDA approved for drug delivery
poly (lactic-co-glycolic acid) → can potentially be used for gene delivery also
benefits
increasing transfection efficiency
navigating through the multiple biological barriers
naked DNA
muscle cells were first shown to be able to take up naked DNA and express proteins
99% of injected DNA is degraded by extracellular nucleases
large amounts of DNA required
very low gene transfer efficiency leads to low and transient protein expression
NOT immunogenic
useful when the effect of the expressed protein is naturally amplified (such as to activate immune system via cytokines or antigens)
basically when even small amounts of protein can have a big effect
increase transduction efficiency by electric impulse or ‘gene gun’
biolistic gene gun
uses kinetic energy to deliver nucleic acids inside cells
DNA is coated on gold or tungsten particles
5-10% transduction efficiency
only be used for exposed targets such as skin for local transfection that can withstand pressure
NOT useful for deep tissues
e.g. skeletal myotubules that are hard to transduce with viral vectors due to lack of receptors can be transfected with gene guns
summary of viral and non-viral gene delivery system
viral vectors
pros
high transfection efficiency
natural tropism → ability to infect different cell types
evolved mechanisms for endosomal escape
viruses have evolved ways to avoid being destroyed inside cell’s endosomes
natural transportation mechanism of DNA into nucleus
cons
strong immune rxns against vial proteins prohibit multiple admins
possibility of chromosomal inserting and proto-oncogene activation
complicated synthesis process
toxicity, risk of contamination of live virus
limitation of gene size
non-viral vectors
pros
low immunogenicity
can be made to be non-toxic
easy to synthesis → quality control for mass production
potentially targetable
NO limit on plasmid size
No integration → can be admin as drugs
cons
low transfection efficacy
NO natural tropism, endosomal escape or nuclear transport mechanisms
process of clinical trials

clinical trials of gene therapy
1972 → concept of gene therapy considered as form of treatment
1975 → first organ cell for therapy isolated from fetus
1985 → studies report safe use of fetal organ cell in patient
1990 → first gene therapy trial in humans
1999 → death of jesse gelsinger in AAV therapy trial
2003 → first gene therapy clinical trial for parkinson’s disease is initiated
2012 → jennifer doudna and emmanuelle charpentier developed the CRISPR-cas9 gene editing system
2017 → FDA approved first gene therapy in U.S.; kymriah, CAR-T cell therapy, was approved
2023 → first CRISPR therapy seeking FDA approval
gene therapy clinical trials approved or initiated 1989-2023
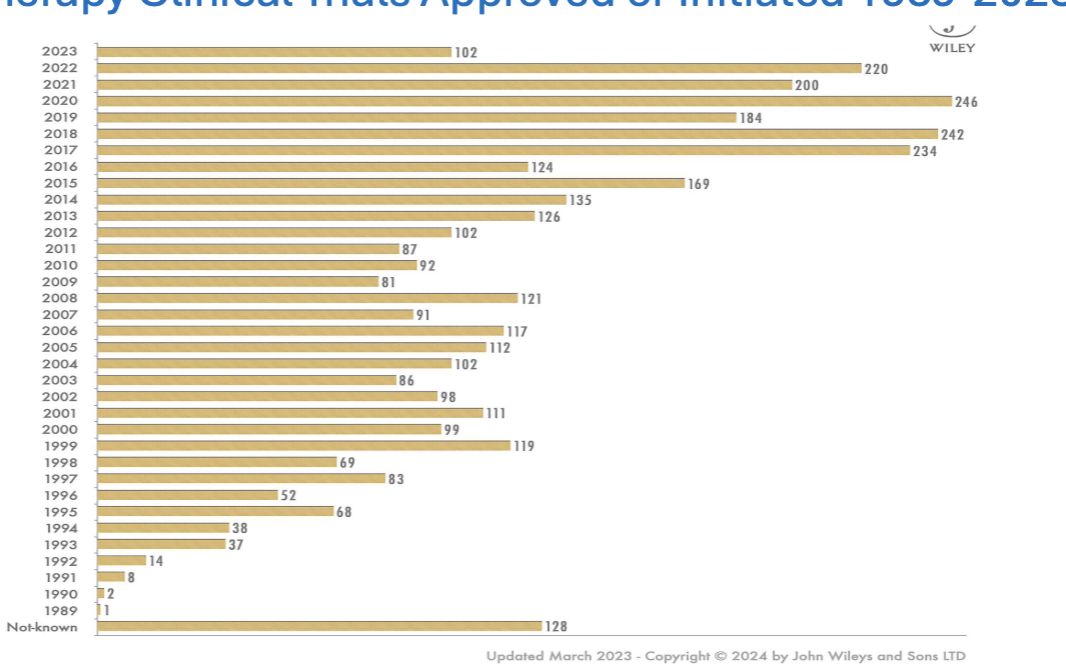
total number of gene therapy trials currently
3900
most in phase I
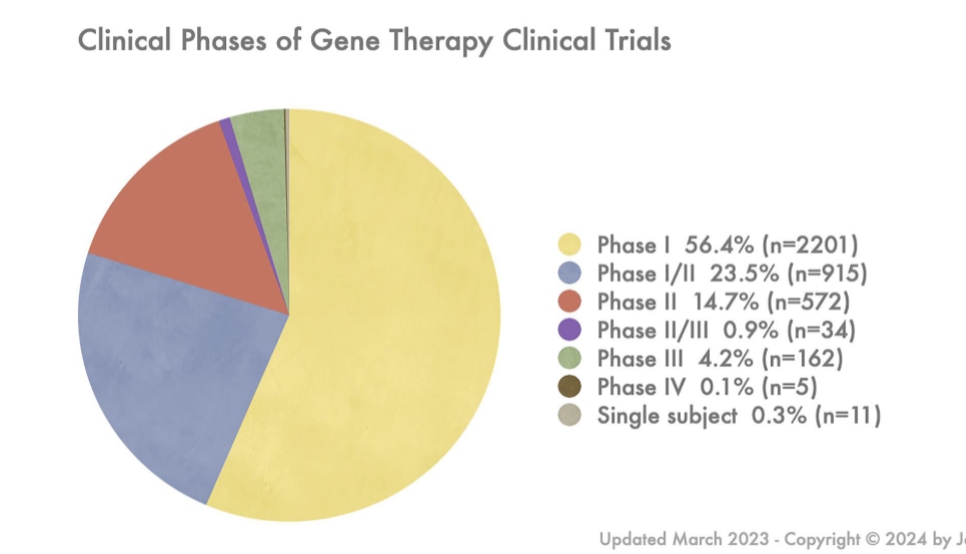
gene types transferred in gene therapy clinical trials
mostly receptor followed by antigen
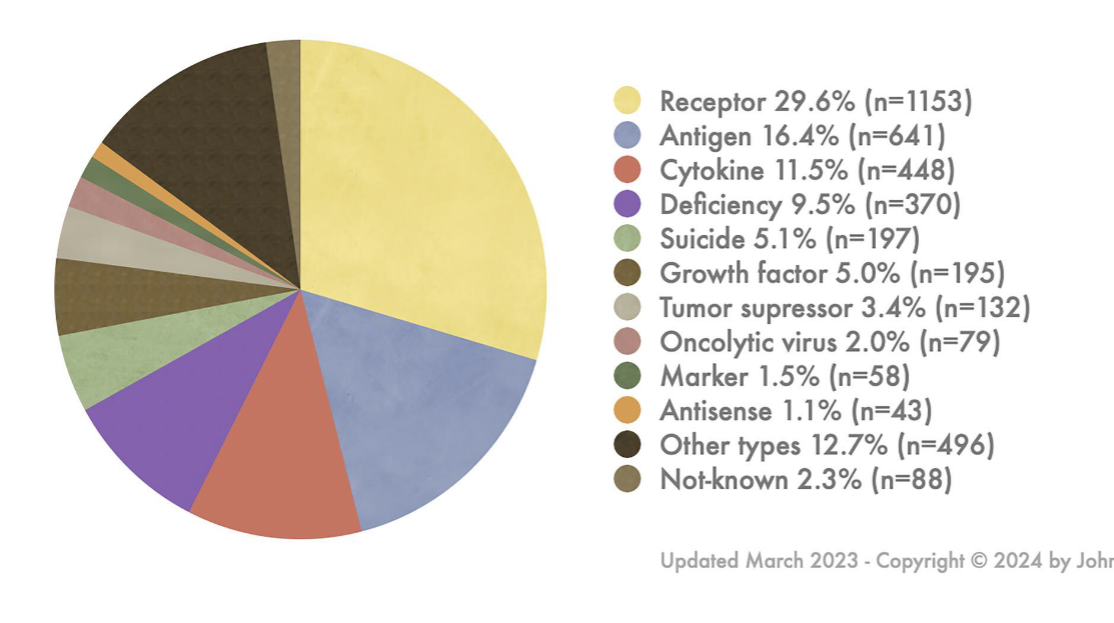
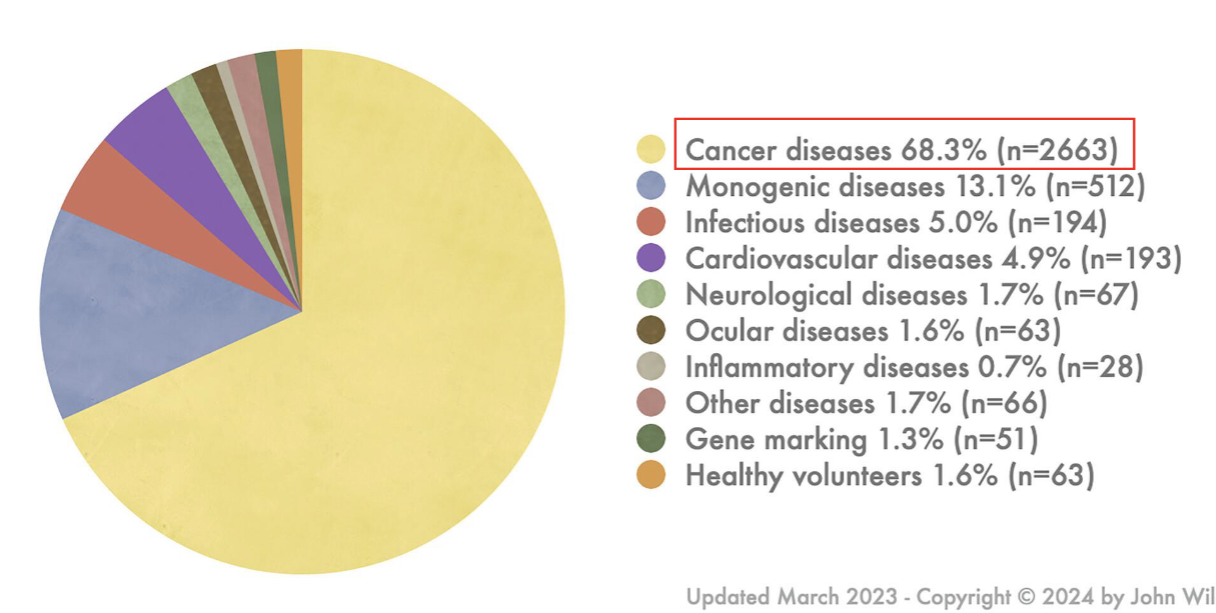
indications addressed by gene therapy clinical trials
mostly cancer
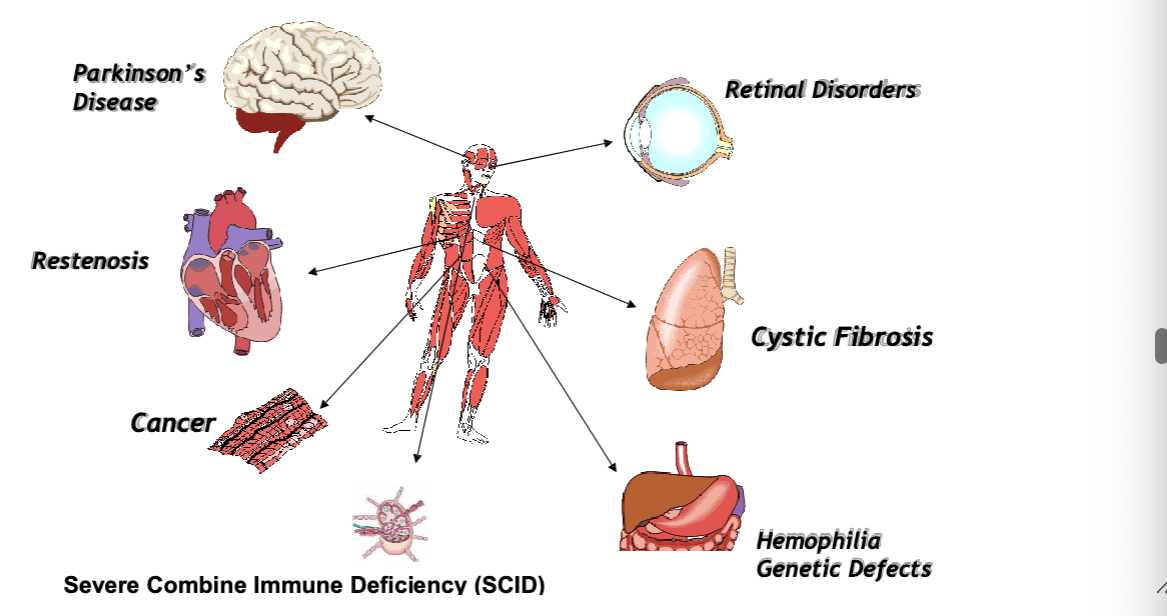
clinical application — case studies
parkinsons
restenoisis
cancer
severe combine immune deficiency (SCID) (bubble boy disease)
retinal disorders
CFR
hemophilia genetic defects
clinical feature of ADA-SCID (adenosine deaminase deficiency)
ADA deficiency = cause of approx 20% of severe combined immunodeficiency (SCID)
pts have multiple severe opportunistic infections usually beginning in infancy
inherited as autosomal recessive condition
rare, <1/100,000 births
usually fatal in 1st year of life if untreated
selective toxicity to lymphocytes from accumulation of metabolites of deoxyadenosine, esp dATP
rational for considering ADA-SCID for initial gene therapy trials
normal human ADA gene had been cloned
a single gene defect leads to loss of enzymatic function
HLA-matched allogenic bone marrow transplantation completely corrects the disease
treatment for those patients who lack HLA-matched bone marrow donors is with recombinant ADA protein
corrected cells have selective growth advantage in vivo
small amount of ADA activity is sufficient to correct disease
first human gene therapy trial - 1990
2 girls (4 and 9) with ADA-SCID = first gene therapy recipients
ADA gene delivered into patient lymphocytes using retroviral vectors; ex vivo therapy
patients continued to receive ADA protein replacement therapy
immune systems restored
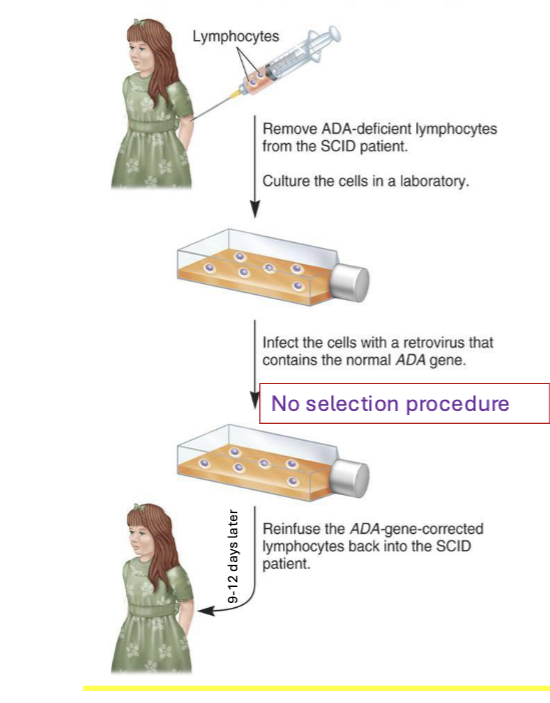
process
remove ADA-deficient lymphocytes from SCID patient → culture the cells in a lab
infect the cells with a retrovirus that contains the normal ADA gene
no selection procedure
reinfuse the ADA-gene-corrected lymphocytes back into the SCID patient
both girls had the transgene years after treatment and exhibited robust immune systems
a case study for failed gene therapy clinical trials
jeese gelsinger, relatively fit 18yr old with ornithine transcarbamylase deficiency (OTC) died on sep 17,1999 in a gene therapy trial at upenn, 4 days after he was administered a massive dosage of 38 trillion genetically altered adenovirus particles carrying the OTC gene directly into the hepatic artery in the liver
what went wrong?
the vector invaded organs other than the intended target
only 1% of the transferred genes reached the target cells
harsh immune response was triggered (may be an undetected genetic condition or a latent parvovirus infection)
X-linked severe combined immunodeficiency (SCID)
40-50% of SCID cases
caused by deficiency in y subunit of cytokine IL-2 receptor (IL2RG gene)
the y subunit of IL-2 receptor is also called common y chain (cy) as it is a subunit common to the receptors for 5 other cytokines also:
IL-4
IL-7
IL-9
IL-15
IL-21
received an infusion of autologous bone marrow-derived CD34+ cells transduced with the y chain-IL2R containing retroviral vector
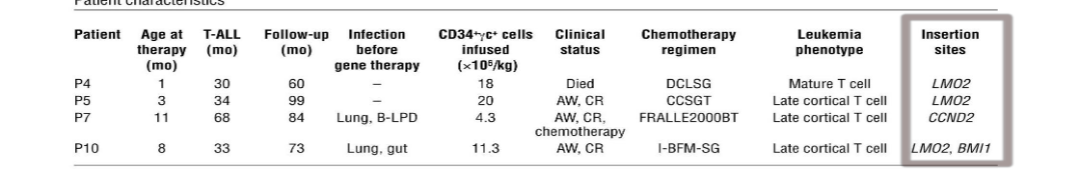
gene therapy caused 4 patients to develop leukemia 2-4 years after treatment
possible reasons for leukemia development
something about the vector virus made it integrate at oncogenic sites (LMO2)
the transferred gene IL2RG itself is oncogenic
genetic background of patient makes them predisposed to cancer (siblings of some patients developed cancer)
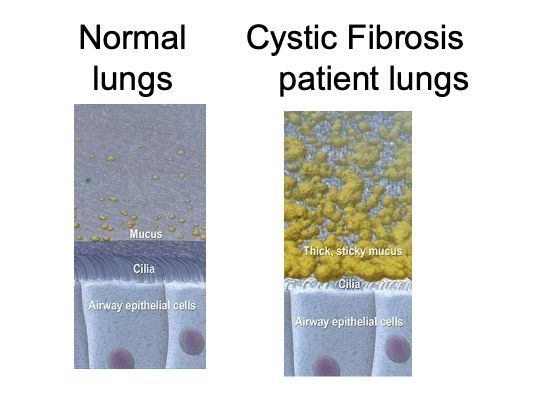
cystic fibrosis (CFR)
effects 1 in 3300 caucasians and 1/25 are carriers (most common hereditary disease in caucasians)
gene
CFTR
name of protein
cystic fibrosis transmembrane conductance regulator
locus
7q31.2
size
CFTR gene consists of 27 exons (coding regions)
gene (total # of bps of exons + introns) is over 250,000 bps (250 kbp)
after introns are spliced out, mRNA = 6100 bps long and is translated into the 1480 AA sequence of CFTR protein
protein function
normal CFTR protein is a channel protein found in membranes of epithelial cells
major symptoms
impaired lung function
chronic respiratory infections
airway inflammation
CFTR gene codes for an ABC transporter class ion channel proteins that conducts chloride and thiocyanate ions across epithelial cell membranes
mutations of the CFTR gene affecting Cl- ion channel function → lead to dysregulation of epithelial fluid transport in the lung, pancreas and other organs → results in CFR
why is CFR an ideal candidate for gene therapy?
single gene defect
recessive condition (heterozygotes normal, suggesting no gene dosing effects, means small changes in gene expression are unlikely to cause harm)
main pathology = lung which is accessible for treatment
progressive disease with virtually normal phenotype at birth thereby providing a therapeutic window from symptom to treatment
protein replacement therapy in lungs NOT available and may NOT be possible for a membrane protein
gene therapy might be the only option
what are the requirements for CFR gene therapy to work?
adequate carrying capacity by gene delivery system
to be undetectable by the immune system
to be non-inflammatory
to be safe to patients with pre-existing lung inflammation
to have an efficiency sufficient to correct the cystic fibrosis phenotype
to have long duration of expression or the ability to be safely re-administered
gene therapy for CFR
first trials in NY in 1993 using adenoviral vector were unsuccessful due to significant immune rxns and inflammation in the lungs
clinical trial currently ongoing by the UK cystic fibrosis gene therapy consortium: CFTR gene introduced via aerosol to lungs using cationic liposomes
136 patients aged 12 and above were randomly assigned to either nebulised pGM169/GL67A (gene therapy) or saline (placebo) at monthly intervals over 1 year; lung function was evaluated using a common clinical measure FEV1 (amount of air you can force from your lungs in one second)
clinical trials reached its primary endpoint with patients who received therapy having a significant if modest benefit in lung function compared with the gene therapy, FEV was 3.7% greater compared to placebo
trial is the first ever to show that repeated doses of a gene therapy can have a meaningful effect on the disease and change the lung function of patients
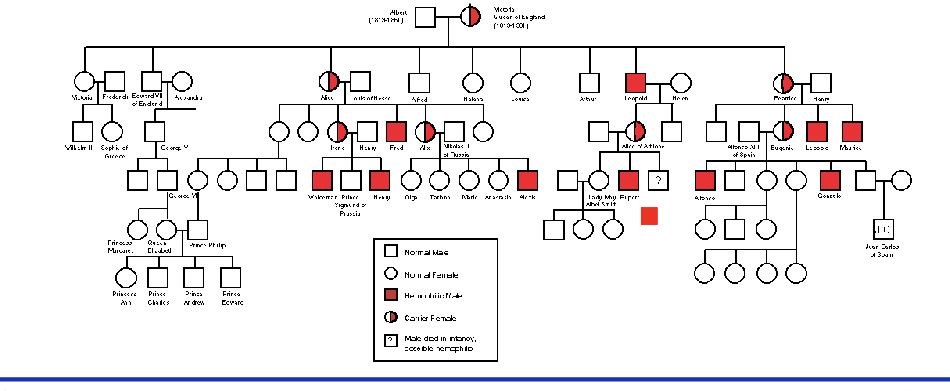
hemophilia B (christmas disease)
X linked genetic disorder
affects 1/100,000 male births
abnormal bleeding due to defective coagulation factor IX
mutation in factor IX now know to be cause of ‘royal disease’
queen victoria and many of her descendants carried what was once called ‘royal disease’
gene therapy for hemophilia B
classification (factor IX activity)
normal → 100% (factor IX activity) → 5 mg/mL (factor Ix concentration)
mild → 5-50% → >250 ng/mL → bleed as result of surgery or major injury
moderate → 1-5% → 50-250 ng/mL → bleed less frequently, once a month
severe → <1% → <50 ng/mL → bleed 1-2 times per week, spontaneous, no reason
40% of cases are severe
effective care can be achieved with levels of 85-100 ng/mL factor IX in blood; in other words → symptoms can be significantly reduced by expression of only 1.5-2% of normal levels of factor IX
factor IX cDNA gene = 1.5 kbp
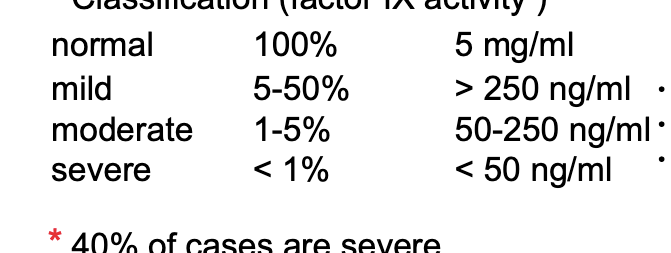
current treatment for factor IX deficiency
infusion of factor IX protein IV
concentrated from donated human blood
improved manufacturing and production of rfactorIX has reduced complications of contaminations associated with using a blood product
factor IX half life → 18-24 hrs
in america, infusions given usually only in management of crisis situations
can cost up to $20 million over the lifetime of a patient
gene therapy which obviates IV infusions would advance care both clinically and economically
long-term expression of factor IX after gene therapy using AAV
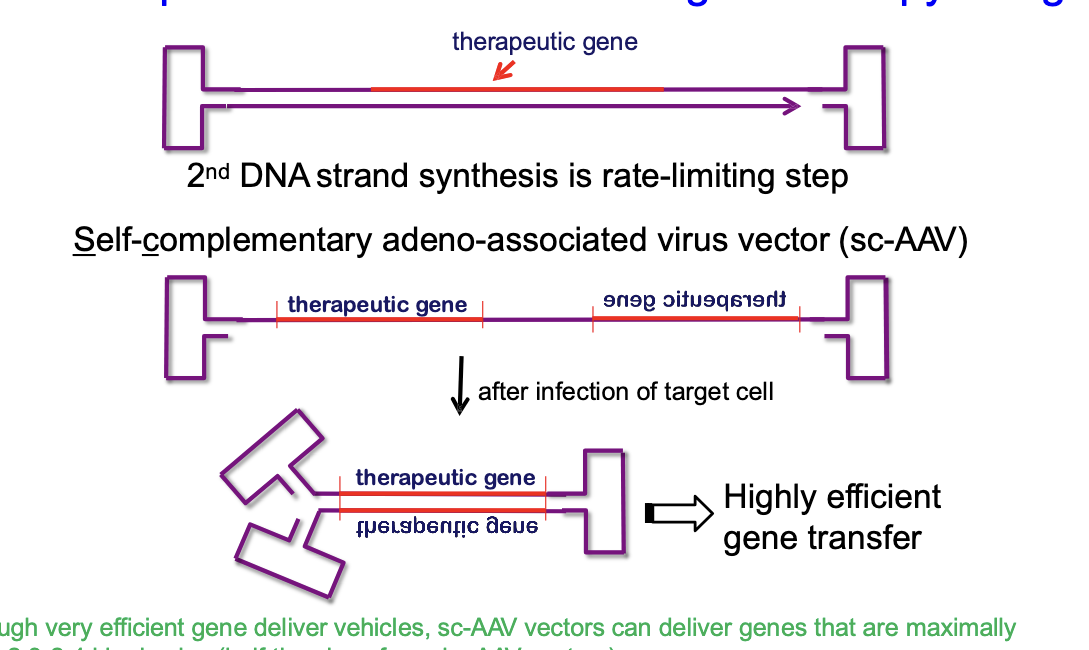
AAV normally delivers a single-stranded DNA version of the therapeutic gene
after virus infects the target cell, cell has to synthesize the 2nd DNA strand to make it double stranded before gene can be used → second strand synthesis is slow (rate limiting step)
solution
self-complementary adeno-associated virus vector (sc-AAV)
instead of delivering just 1 DNA strand, sc-AAV delivers both strands already connected together → once inside the cell, the 2 strands fold and bind to form double stranded DNA quickly → allows the therapeutic gnee to start working faster and more efficiently
although very efficient gene deliver vehicles, sc-AAV vectors can deliver genes that are maximally only ~2.3-2.4 kbp in size (half the size of regular AAV vectors)
hemophilia A
most common type of hemophilia
5x more common than hemophilia B
X-linked disorder
factor VIII gene near factor IX on X chromosome
gene (cDNA) = ~9kbp
good target for gene therapy as even a low level of secreted protein expression can prevent symptoms
other applications of gene therapy
hereditary blindness: leber’s congenital amaurosis (LCA)
caused by mutation in RPE65 gene
eye disorder that primarily affects the retina
4 independent clinical trials with AAV2-containing RPE65 reported improvement in vision with none being associated with vector-related adverse events
lysosomal storage diseases
more than 50 diseases are considered lysosomal storage disorders
most common: gaucher disease, fabry disease, hunter syndrome, hurler syndrome, tay-sachs disease, pompe disease
rare, inherited conditions occur when the body does NOT produce the enzymes needed to help break down certain substances such as fats, sugars or proteins
parkinsons
cell death in brain region called ‘substantia negra’
responsible for making neurotransmitter dopamine
currently treated with exogenous dopamine systemically which inefficiently crosses BBB and has side effects
gene transduction of tyrosine hydroxylase → enzyme that synthesizes dopamine
strategy for the rationality in the gene therapy trials in parkinson’s
parkinsons has a complex pathophysiology that is by no means fully understood and involves multiple brain structures and signaling pathways
3 broad approaches to selection of a therapeutic target
restoration of dopamine synthesis in the dorsal striatum
modulation of activity in the basal ganglia downstream of the striatum
modification of disease progression by neuroprotection
vectors associated with gene therapy for parkinsons
over-expression of GDNF (glial cell line-derived neurotrophic factor) mediated by lentiviral vector has conferred some protection of the nigrostriatal dopamine terminal against toxic insults
ad-GDNF can protect dopaminergic neurons and improve dopamine-dependent behavioral function in young rats with progressive 6-OHDA lesions of the nigrostriatal projection
AAV-2 when administered locally transduced only neurons within the CNS and is particularly efficient in brain regions known to be involved in the pathophysiology of parkinsons such as globus pallidus and substatia nigra
long term safety and tolerability of prosavin, lentiviral vector based gene therapy
dose escalation, open-label → phase 1/2 trial
phase 3 trial → called exPDite-2 study, expected to begin in the first half of 2025, will enroll 102 pts to assess efficacy and safety over 78 weeks
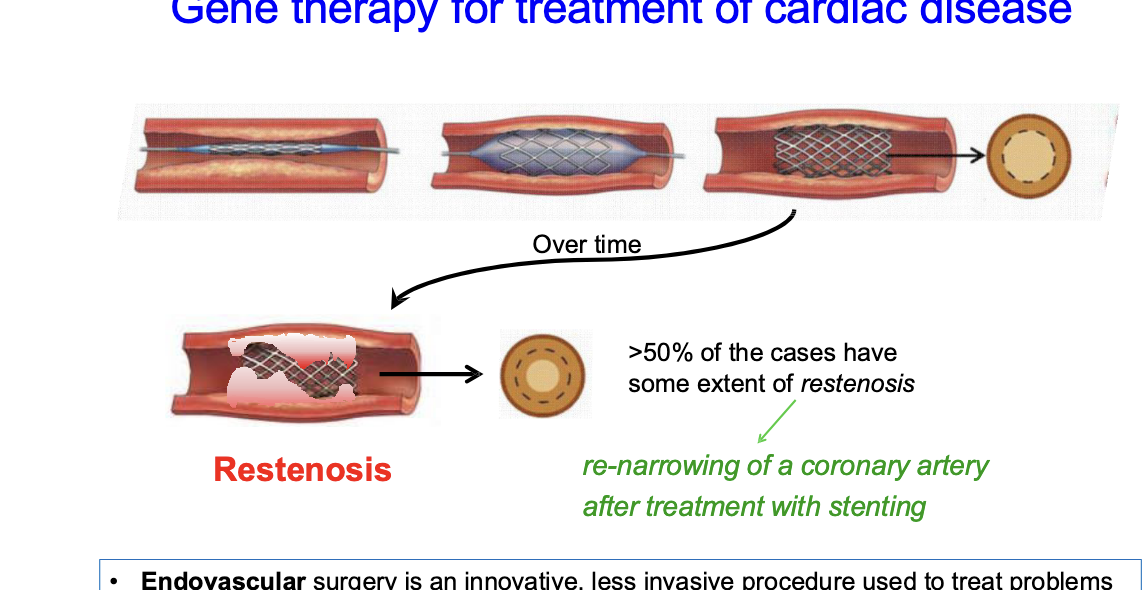
gene therapy for treatment of cardiac disease
endovascular surgery = innovative, less invasive procedure used to treat problems affecting the blood vessels
blocked artery is opened using a stent → keeps artery open, restoring blood flow
over time, >50% of patients, artery narrows again in process called restenosis = common adverse event of endovascular procesdures
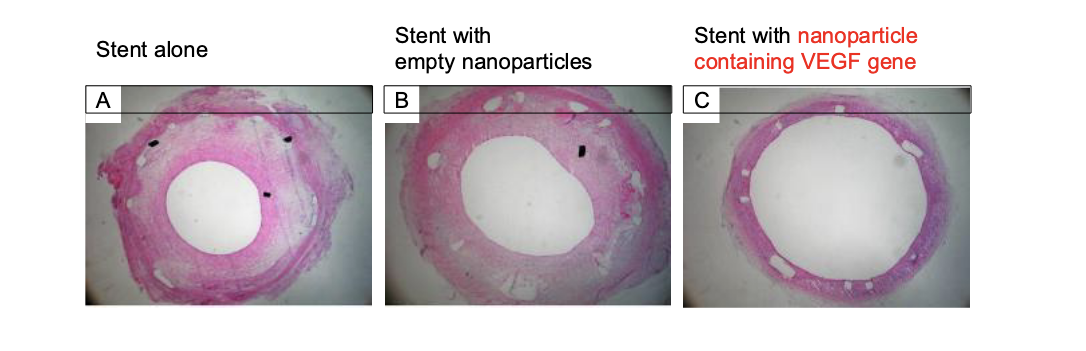
gene-eluting stents for preventing coronary restenosis
stent coated with PLGA-nanoparticles containing vascular endothelial growth factor (VEGF) gene in animal model system
keeps artery OPEN
other potential genes to treat restenosis
PCNA (proliferating cell nuclear antigen)
NOS (nitric oxide synthase)
gene therapy in cancer
engineer T cells to recognize tumor antigens like CEA, NY-ESO-1, CD19
immunopotentiating genes (IL2, B7, GM-CSF) introduced into tumor cells to increase immune rxn to tumor
restoring tumor suppressor genes like p53, BRCA1, Rb that are mutated in cancer cells
anti-sense therapy to turn OFF oncogenes/replication genes
suicide genes → implant herpes simplex thymidine kinase into tumor cells
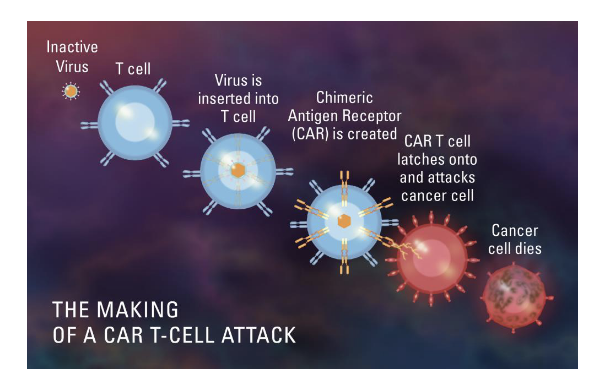
immunotherapy: CAR T cell therapy
enhance the natural cancer-fighting ability of patients’ own T cells
sample of patients T cells is collected and mixed with viruses carry several specific genes
viruses deliver these genes to the T cells nuclei where they are incorporated into the cell’s DNA
the genes cause the T cells to express a special protein called a chimeric antigen receptor, or CAR, on their surface
CAR directs the T cell to the tumor cell using a specific ‘address’ and the CAR T cell is then equipped to rapidly destroy the cancer cell
when the CAR T cells are infused into the patient → they seek out tumor cells and then proliferate to generate many more cancer-killing cells
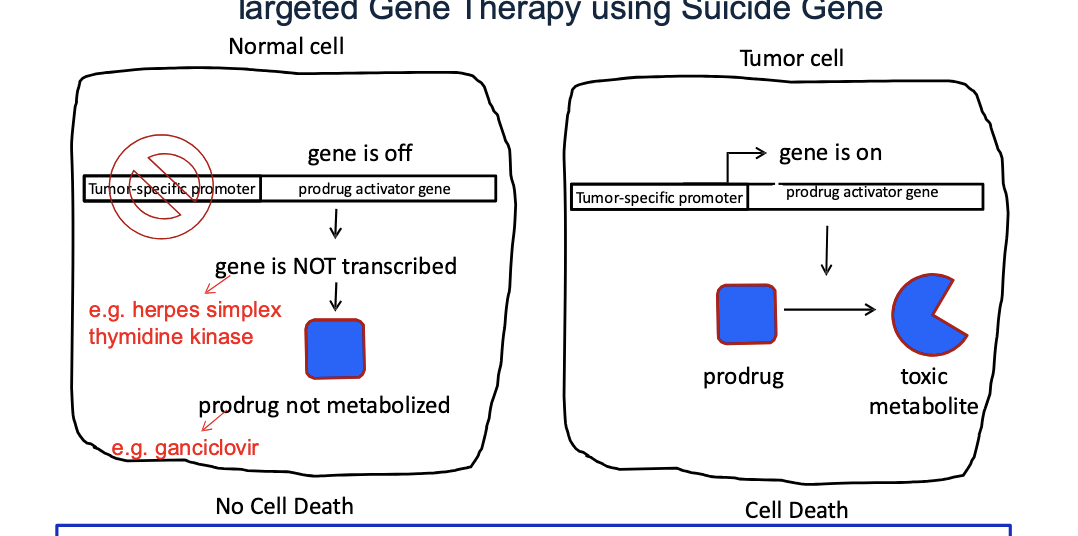
targeted gene therapy using suicide gene
normal cell
prodrug activator gene (e.g. herpes simplex thymidine kianse) is OFF b.c the tumor specific promoter is inactive in normal cells → gene is NOT transcribed → prodrug (e.g. ganciclovir) NOT metabolized → NO cell death
tumor cell
tumor specific promoter is ON → turns the prodrug activator gene ON → enzyme converts prodrug to toxic metabolite → cell death
basic concept of suicide gene therapy = introduction of viral or bacterial genes into tumor cells which convert a non-toxic prodrug → toxic one
gene-directed enzyme-producing therapy (GDEPT) → uses a gene taken from the cancer cell and then modified with other genes to form enzymes that are harmless to healthy cells but in cancer cells will activate the prodrug to turn into toxic metabolite that will kill cancer cells
virus-directed enzyme-prodrug therapy → uses a virus, such as herpes simplex or cold virus, as the carrier, or vector, to deliver the modified genes to cancer cells
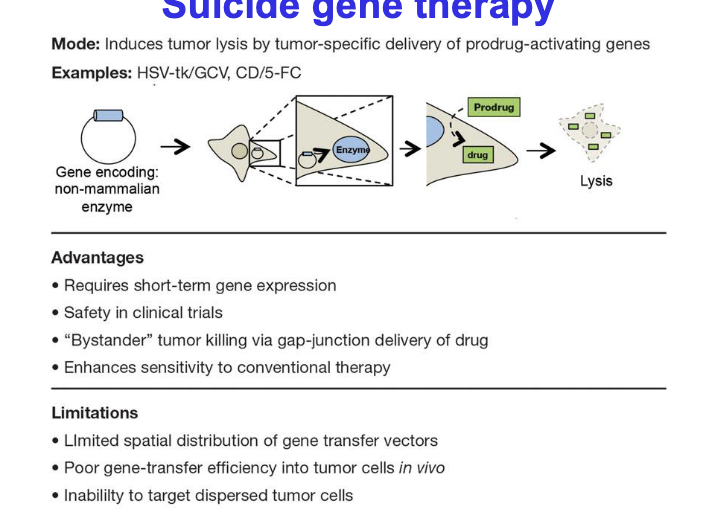
suicide gene therapy
mode → induces tumor lysis by tumor-specific delivery of prodrug-activating genes
examples
HSV-tk/GCV
CD/5-FC
advantages
requires short-term gene expression
safety in clinical trials
‘bystander’ tumor killing via gap-junction delivery of drug
enhances sensitivity to conventional therapy
limitations
limited spatial distribution of gene transfer vectors
poor gene-transfer efficiency into tumor cells in vivo
inability to target dispersed tumor cells
cytosine deaminase gene (CD) of escherechia coli = converts the pro-drug 5-fluorocytosine (5-FC) → 5-fluorouracil (5-FU)
herpes simplex virus thymidine kinase gene (HSC-tk) = converts ganciclovir (GCV) to ganciclovir monophosphate, converted by the cancer cells’ enzymes to ganciclovir triphosphate
suicide gene therapy has demonstrated limited clinical efficacy for treatment of malignant glioma (aggressive brain tumor)
advances in gene editing technologies
in vivo CRISPR editing
researchers have begun editing genes directly inside the human body
for instance, intellia therapeutics and regeneron pharmaceuticals have developed treatemnts that use lipid nanoparticles to deliver CRISPR components to the liver, targeting diseases like transthyretin amyloidosis
early results show significant reductions in disease causing proteins
base and prime editing
next generation CRISPR techniques such as base and prime editing allow for precise correction of a single-letter genetic mutations WITHOUT cutting the DNA
these methods are being explored for treating conditions like progeria and other monogenic diseases → offers potential for safer and more accurate therapies
CRISPR technology
simple yet powerful tool for editing genomes, allows researchers to easily alter DNA sequences and modify gene function
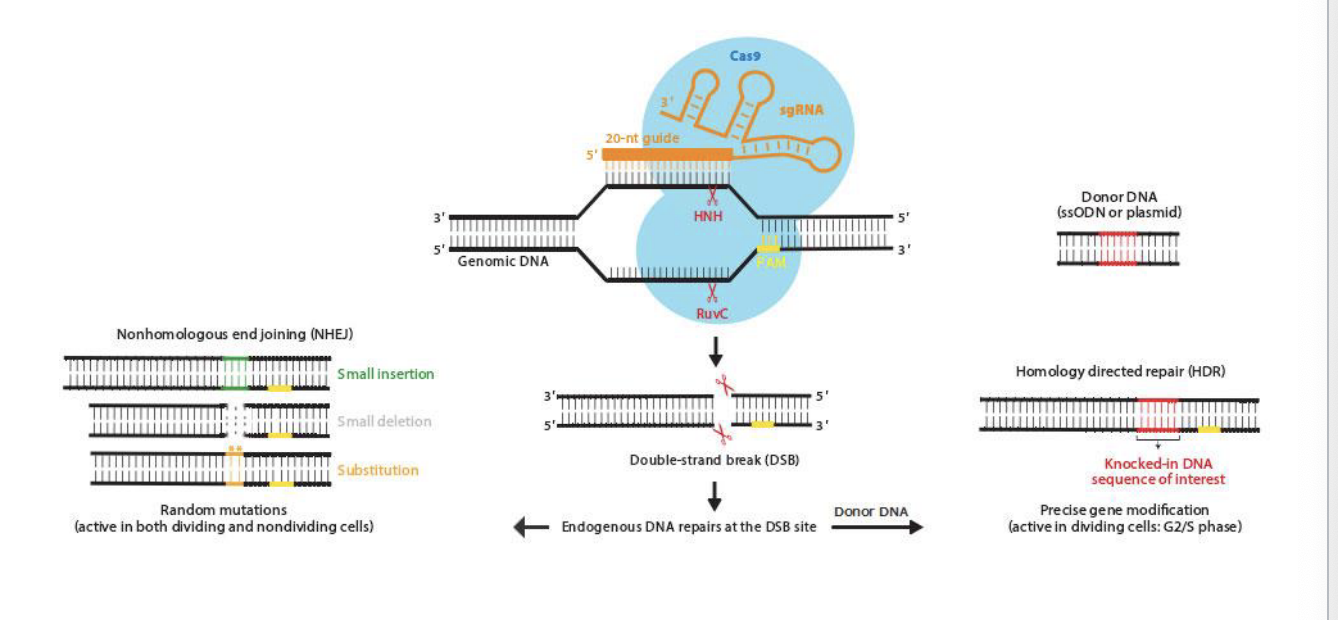
components of the CRISPR-Cas9 system
2 core components
RNA guided DNA endonuclease Cas9
chimeric single guide RNA (sgRNA)
the sgRNA, which has an invariant scaffold region and a spacer region, is derived from CRISPR RNA (crRNA) and transactivating crRNA (tracrRNA)
sgRNA binds to Cas9 and directs it to the locus of interest by a 20-nt guide sequence via base pairing to the genomic target
the target sequence in genomic DNA paired to sgRNA sequenced is immediately followed by either NGG or NAG trinucleotide for S. pyogenes-derived cas9 (other cas9 orthologues recognize different PAM) called the protospacer adjacent motif (PAM)
Cas9 only cuts DNA if the target site is followed by a special PAM sequence, which for the common cas0 form s. pyrogenes is NGG or NAG
PAM is located on the immediate 3’ end of the sgRNA recognition sequence but is NOT a part of the 20-nt guide sequence within sgRNA
mechanism of CRISPR-cas9 mediated genome engineering
cas9 enzyme uses sgRNA with a 20-nt guide to find and bind the matching sequence in the genomic DNA → once bound, cas9 cuts both strands at that location using:
HNH → cuts one strand
RuvC → cuts other strand
result = double strnad break
cell repairs the break in one of 2 ways
nonhomologous end joining (NHEJ)
quick fix, NO template needed
cells glue the broken ends together but introduces random mutations
small insertion
small deletion
substitution
can disrupt the function of a gene
homology directed repair (HDR)
precise fix, requires a template
scientists provide a piece of donor DNA containing the desired sequence
cell copies this donor DNA into the broken site
precise gene editing or gene knock in
CRISPR technology: potential applications and advantage
altering specific genetic loci thru insertions, deletions, point mutations, and sequence inversions
treating and preventing the spread of diseases and improving crops
transform medicine, enabling us to NOT only treat but also prevent many diseases
system was recently modified to act as a genome regulator by tethering effector domains to Cas9 or guide-RNA and as a visualization tool by fusing with marker molecules
CRISPR: potential disadvantages
philosophical dilemma
ethical concerns about germline gene editing which are responsible for passing genes on to the next generation
safety
accuracy
off target effects lack of specificity in targeting and incomplete targeting
particularly important issue when it comes to the use of technology for applications directed towards human health
another issues is that once an organism, such as plant or insect, is modified, they are difficult to distinguish from the wild-type and once released into the environment could endanger biodiversity
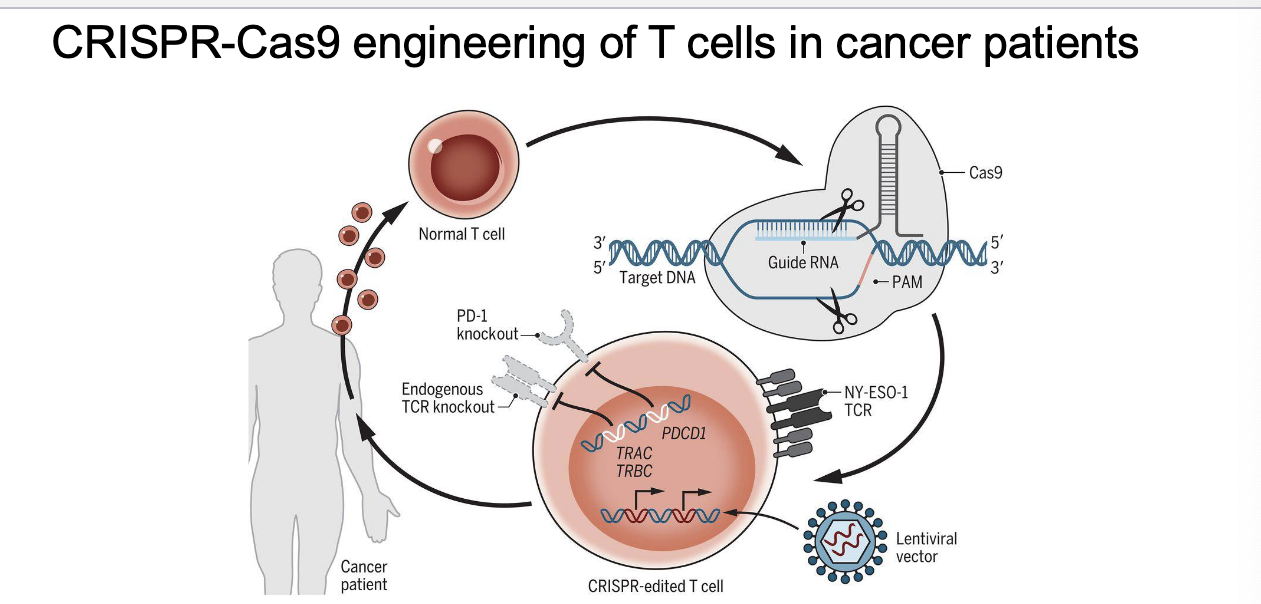
CRISPR engineering of T cells in cancer patients
steps in cycle
collect T cells
CRISPR used to make 3 main changes
PD-1 knockout → PD-1 normally turns OFF T cells and tumors exploit this
TCR knockout → to prevent T cells from accidentally attacking normal tissues
insert new TCR (lentivirus vector) → recognizes cancer-specific antigen and helps T cells find and kill cancer cells
return edited T cells into patient
stay active → b/c PD-1 is gone
only target cancer → new TCR
avoid attacking healthy cells → OG TCR removed
disrupts 3 genes (TRAC, TRBC, and PDCD1)
cancer targeting transgene, NY-ESO-1, introduced to recognize tumors
cancer vaccine
this approach involves:
collecting tumor cells from patient
engineering them with genes that cause them to be conspicuous (immune system can detect them easier) to the immune system
re-infusing altered cancer cells into pt along with immune-stimulating compound
potential complications of gene therapy and strategies to mitigate risk
problem
solution
gene silencing — repression of promoter
use endogenous cellular promoters, avoid viral-derived regulatory sequences
genotoxicity — complications arising from insertional mutagenesis
use vectors with safer integration profile (e.g. self inactivating lentiviral vectors)
sequence-specific integration (e.g. genome editing)
phenotoxicity — complications arising from overexpression or ectopic expression of teh transgene
control transgene expression spatially (e.g. endogenous, tissue-specific promoters) and temporally (on/off switch)
immunotoxicity — harmful immune response to either the vector or transgene
carefully monitor T cell reactivity to the vector and transgene to initiate immune suppression if needed
risk of horizontal transmission — shedding of infectious vector into the environment
monitor vector shedding in preclinical models when developing novel vectors
risk of vertical transmission — germline transmission of donated DNA
use of barrier contraceptive methods until vector shedding is negative
approved gene therapies worldwide

major gene therapy approvals (2023-2024)
casgevy
first CRISPR based gene therapy in the U.S> for sickle cell disease
approved for transfusion-dependent beta thalassemia in Jan 2024
edits the BCL11A gene in a patients stem cells to boost fetal hemoglobin production → reducing disease symptoms
roctavian
approved by FDA in june 2023
one-time gene therapy for severe hemophilia A
delivers a functional copy of the factor VIII gene using AAV vector → enables patients to produce their own clotting factor
hemgenix
approved in nov 2022
treats hemophilia B
AAV vector to deliver factor IX gene → reducing need for regular infusions
vyjuvek
approved may 2023
first topical gene therapy
treats wounds in pts with dystrophic epidermolysis bullosa
employs a modified herpes simplex virus to deliver COL7A1 gene directly to skin cells
upcoming cell and gene therapies that FDA are reviewing
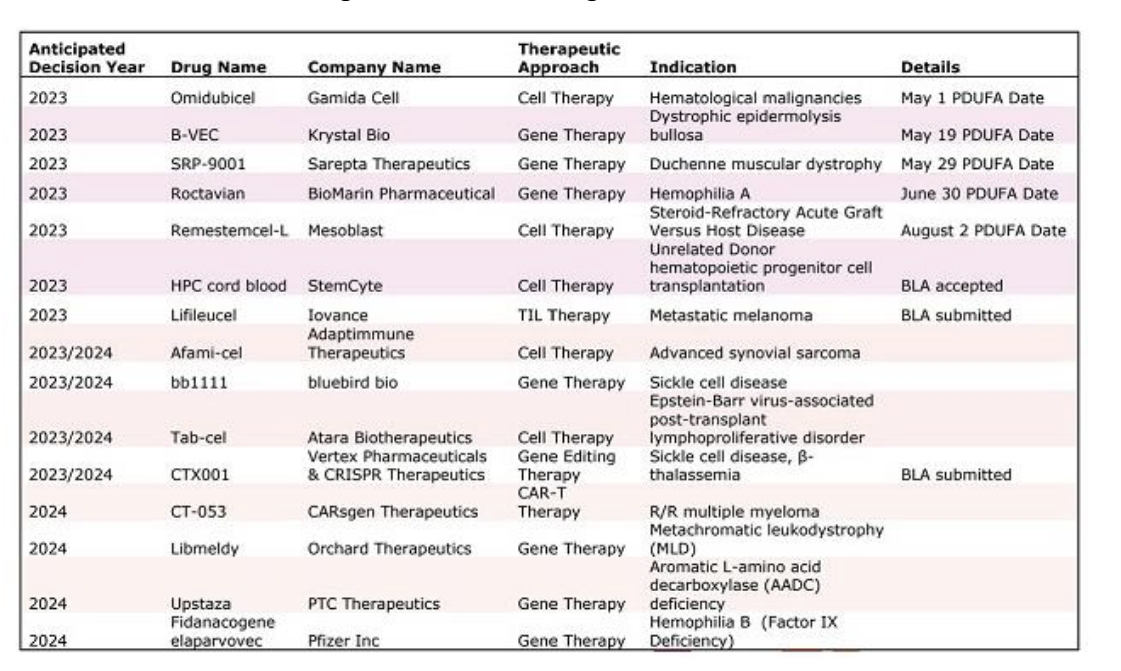
gene therapy: ethical and social issues
who decides which traits are normal adn which constitute a disability or disorder?
will the host cost of gene therapy make it available only to wealthy?
could the widespread use of gene therapy make society less accepting of people who are different?
is it ethical to allow ppl to use gene therapy to enhance basic human traits such as height, intelligence and athletic ability?