Drug Metabolism Basics and Phase 1 Metabolism
1/40
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
41 Terms
3 chemical changes that can occur to drugs
Active drugs can become inactive or another active drug (active metabolite)
Inactive drugs can become active
Therapeutically beneficial drugs can become a toxic agent
2. Phase 2 metabolic pathway
Phase 1 metabolism
These are types of metabolic reactions that occur to drugs that involve oxidation (hydroxylation), reduction, or hydrolysis
-These reactions typically convert drugs to a more polar entity which can then be eliminated in the urine or bile
-It's also possible that they go through phase 2 metabolism first
-Metabolism most often occurs in the liver or small intestine
1st pass metabolism
Hepatic and intestinal metabolism of drugs absorbed from the gut after oral administration that enter circulation
-After drugs go through the stomach they will go through the portal vein which passes through the liver and some drug metabolism will occur there
-After passing through the liver, the drug will enter systemic circulation
-The small intestine will also metabolize drugs because a lot of drug is absorbed here
Examples of ROAs that avoid first pass metabolism
IV
Transdermal
Sublingual
Intranasal
These routes go directly into systemic circulation which doesn't pass through the liver
2. Flavin monooxygenases (FMOs)
3. Alcohol dehydrogenases (ADH)
4. Esterases
5. Amidases
Flavin monooxygenases (FMOs)
A type of enzyme involved in phase 1 metabolism that involves direct oxidation of nitrogen and sulfur atoms
-This type of enzyme doesn't use heme, but rather, it uses flavin adenine dinucleotide (FAD) prosthetic group
2. Oxidative deamination
3. N,O,S dealkylation
4. N-oxidation
5. Desulfurization
6. Sulfoxidation
7. Reductions
8. Hydrolysis
9. Miscellaneous reactions
2. Alkene epoxidation
3. Benzylic hydroxylation
4. Alkyl hydroxylation
5. Allylic hydroxylation
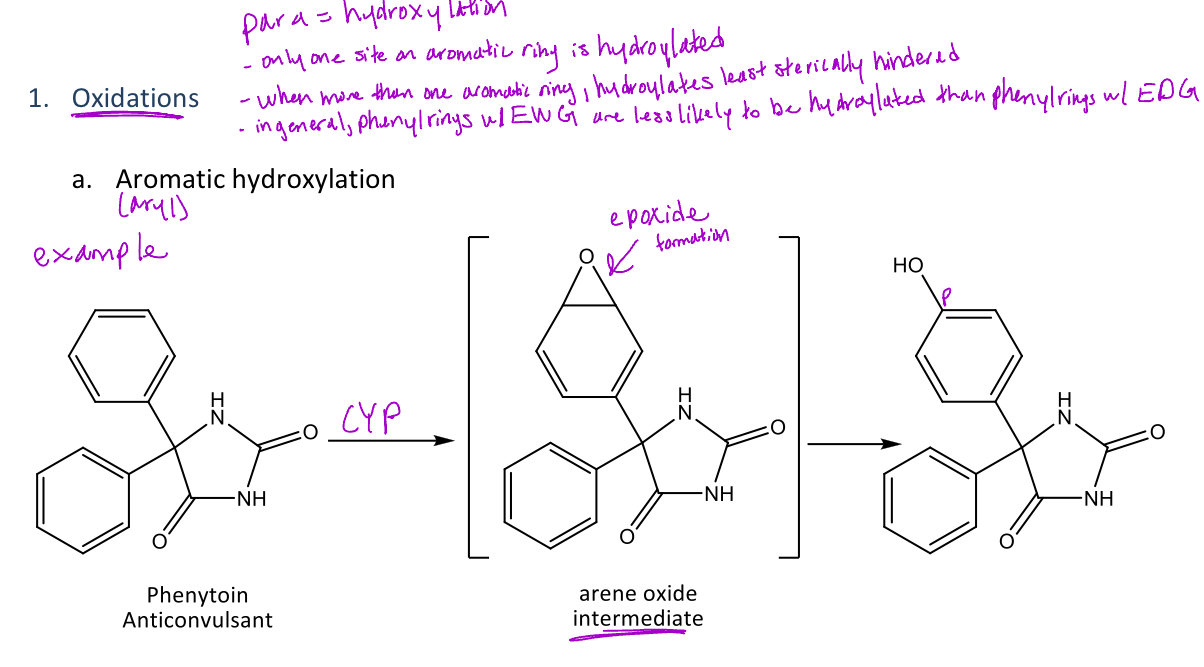
Aromatic (para) hydroxylation
A type of oxidation reaction in phase 1 metabolism where there is an addition of a hydroxyl (OH) group to aromatic ring at the para position
-This is also called para-hydroxylation because the hydroxyl group is added to the para position of the aromatic ring
-This reaction is mediated by the CYP enzyme which will form an arene oxide intermediate
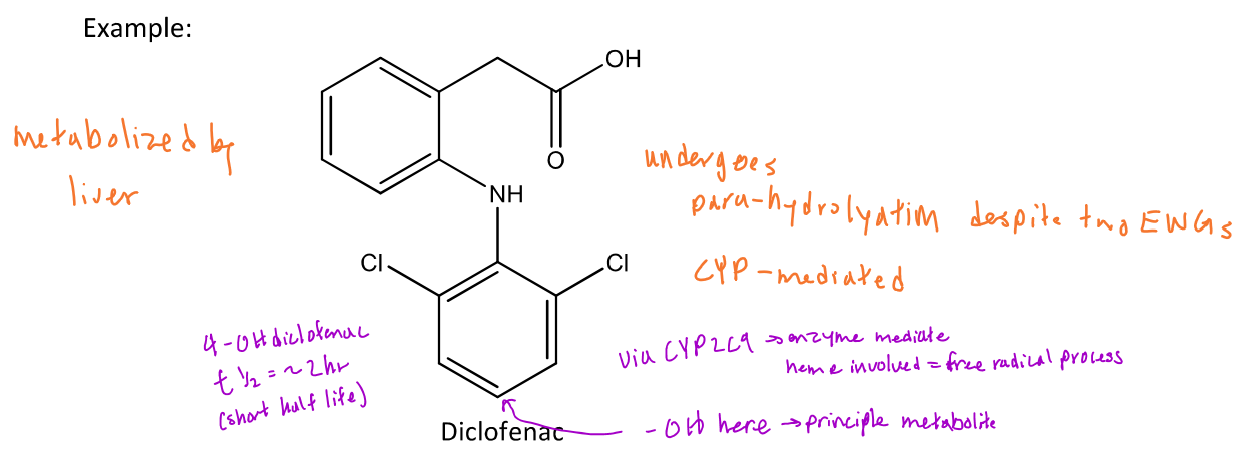
Example of aromatic hydroxylation metabolism (diclofenac)
In diclofenac, aromatic (para) hydroxylation will occur at the para position of each aromatic ring which is enzyme mediated (CYP2C9)
-The final product will be 4-OH diclofenac which has a 1/2 life = 2 hours because of high metabolic clearance
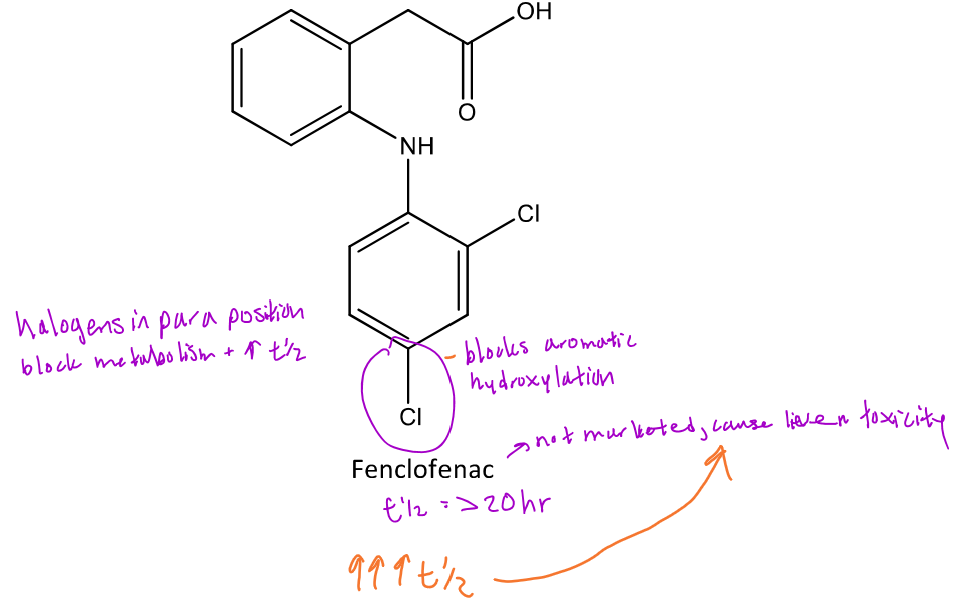
Ways of mitigating para-hydroxylation
Para-hydroxylation can be mitigated by adding a halogen or another group at the para position of aromatic ring
Ex. Fenclofenac has the same structure as diclofenac, *except that it has a Cl at the para position of one aromatic ring which prevents fast metabolism of the drug --> 1/2 life = 20 hours
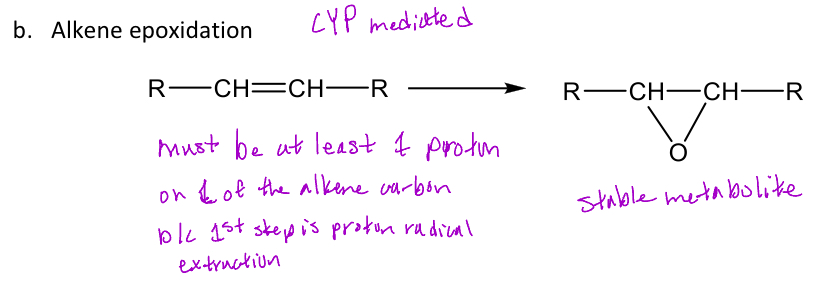
Alkene epoxidation
A type of oxidative reaction in phase 1 metabolism reaction that requires at least 1 Hydrogen on an alkene to form the epoxide at the double bond and is CYP mediated
-The epoxide that's formed isn't an intermediate, but it acts as a stable active metabolite
-Doesn’t occur on aromatic rings
Ex. Carbamazepine (in image) forms an epoxide (phase 1) which is attacked by water to form a transdiol (inactive)
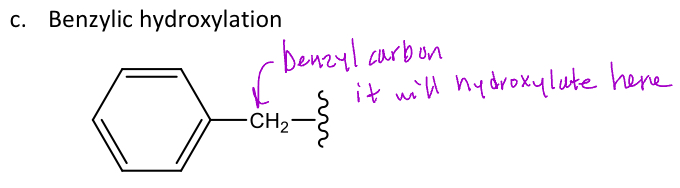
Benzylic hydroxylation
A type of oxidative reaction in phase 1 metabolism that's CYP mediated where hydroxylation will occur at the benzylic site
-The benzylic site is any carbon directly bound to the aromatic ring (doesn't include heteroatoms)
-Typically, benzylic hydroxylation will occur at the least sterically hindered site
-This drug as a 1/2 life = 5 hours
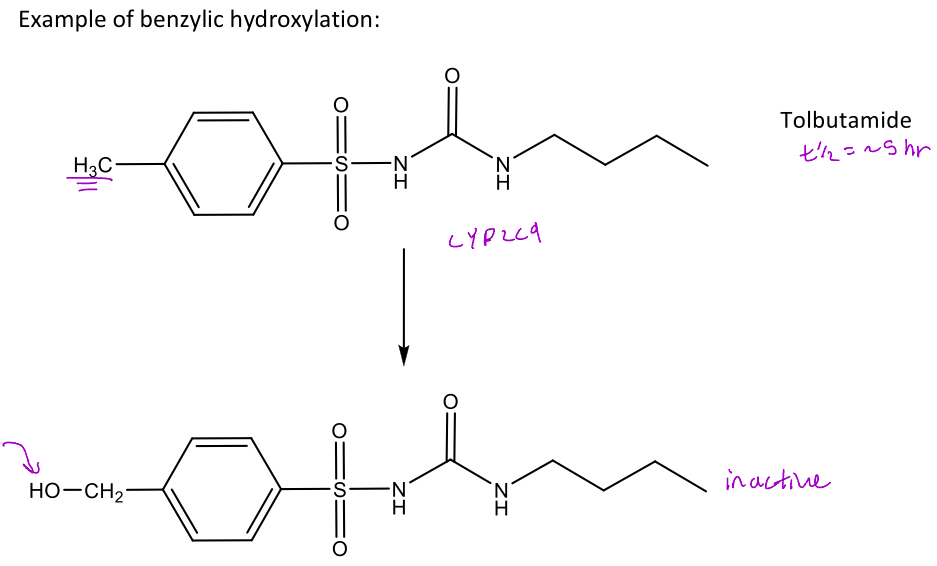
Tolbutamide as an example of benzylic hydroxylation
The first reaction is mediated by CYP2C9 which will add an OH group to the benzylic carbon which can undergo extensive hepatic metabolism and form the inactive compound
It can continue oxdizing the hydroxyl all the way to the aldehyde and then carboxylic acid
Ways of mitigating benzylic hydroxylation from occurring
Benzylic hydroxylation can be mitigated by replacing a benzylic site with a heteroatom
Ex. Chlorpropamide has a similar structure to tolbutamide except it has a chloro group at the para position instead of a methyl group which removes the ability to hydroxylate there*
-This increases the 1/2 = 35 hours -It also prevents para hydroxylation
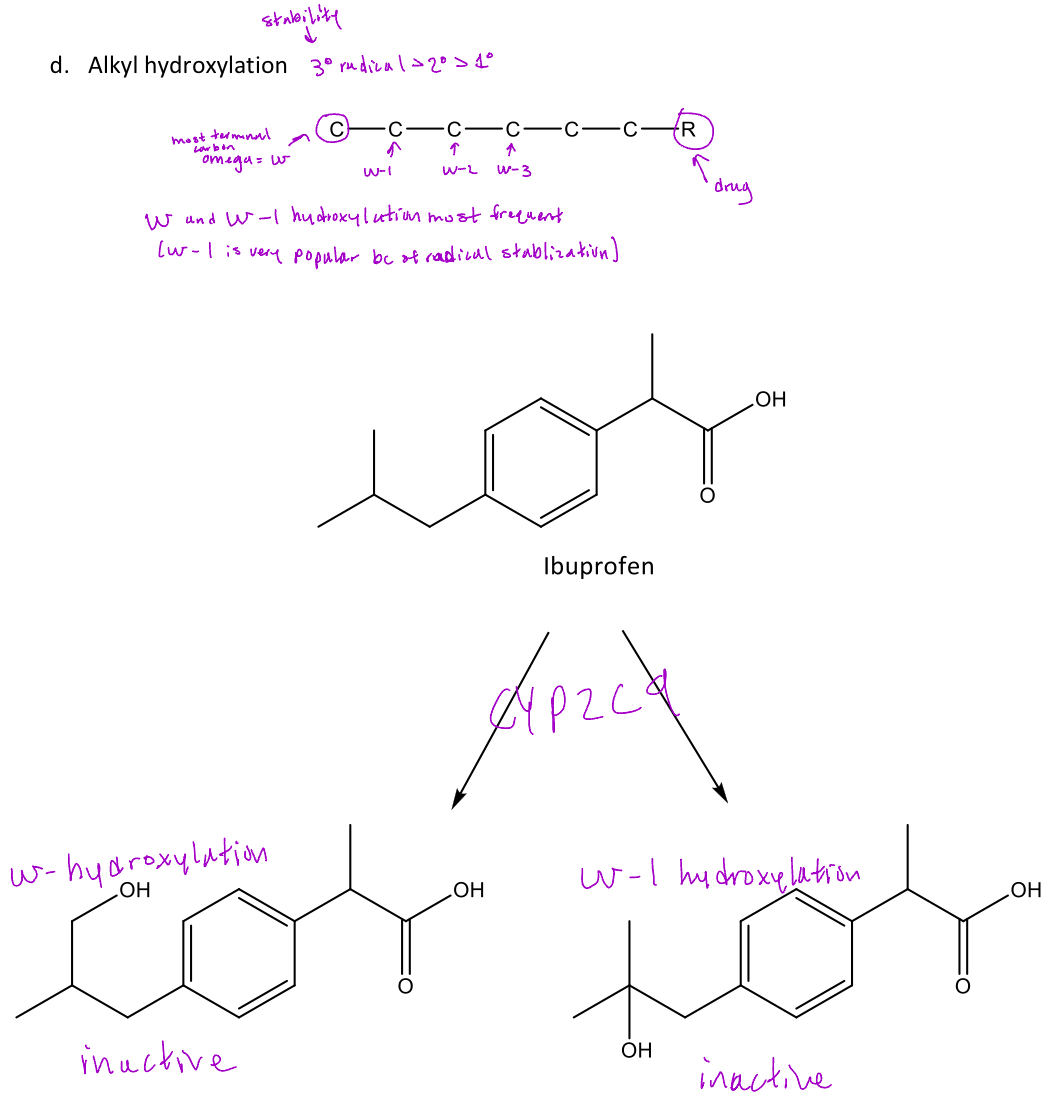
Alkyl hydroxylation
A type of oxidative reaction in phase 1 metabolism which is the addition of a hydroxyl group to the omega (most terminal) carbon or omega-1...
-Omega-1 is most common because it forms a 3° radical which is the most stable
Ex. Ibuprofen will undergo alkyl hydroxylation via CYP2C9 at the terminal carbon (omega) and the the omega-1 carbon
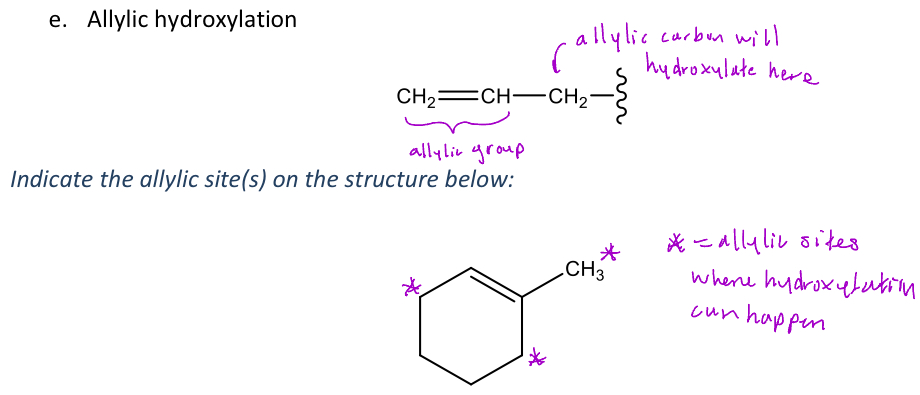
Allylic hydroxylation
A type of oxidative reaction in phase 1 metabolism where the carbon next to an alkene (allylic carbon)
-The allylic carbon will have an OH group added to it, but it's preferred that the allylic carbon isn't sterically hindered
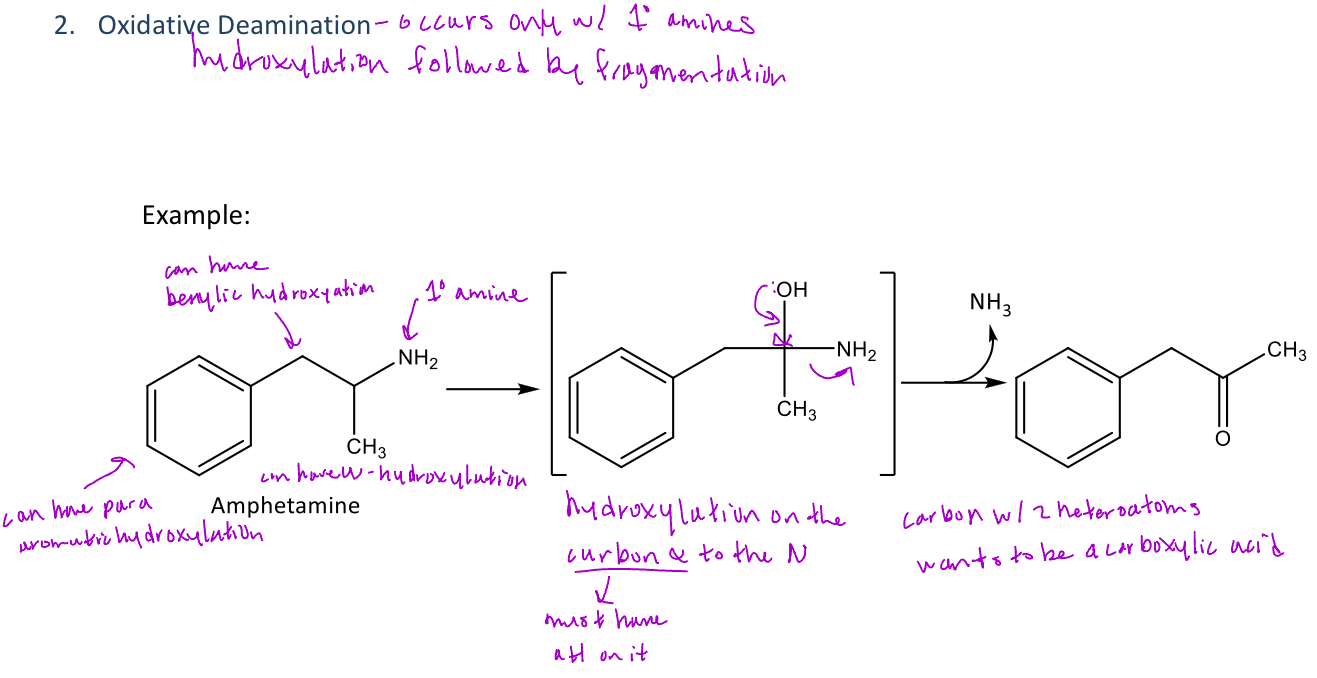
Oxidative deamination
A type of reaction in phase 1 metabolism that only occurs with 1° amines which will go through hydroxylation followed by fragmentation
-Hydroxylation will occur on the carbon that's α to the nitrogen but it must have hydrogen on it
-Fragmentation is the process of removing the NH3 (ammonia) which results in the formation of a ketone
Ex. amphetamine will undergo oxidative deamination and also para-hydroxylation, and benzylic hydroxylation
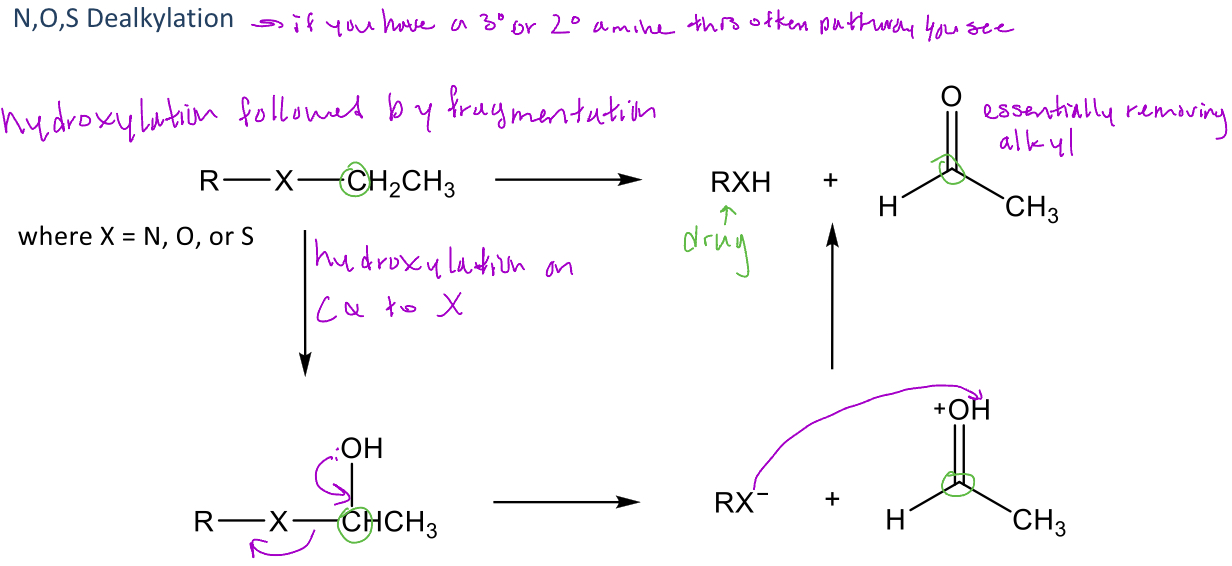
N, O, S dealkylation
A type of reaction in phase 1 metabolism where hydroxylation occurs at the carbon α to an X = N, O, or S (amine, ether, or thioether)
-This reaction follows a similar format to oxidative deamination: hydroxylation followed by fragmentation
-When the groups separate, the compound with the X atom will remove hydrogen from the carbonyl formed
-Occurs w/ 3° amines more than 2° amines
-Can occur with amides
-Alkyl groups that are removed are typically smaller (methyl, ethyl, n-propyl, isopropyl, allyl, and benzyl groups)
Ex. escitalopram will undergo N-dealkylation at the 3° amine which will remove 1 methyl group as formaldehyde (creating S-desmethylcitalopram) but then that compound can be further metabolized through oxidative deamination into S-didesmethylcitalopram
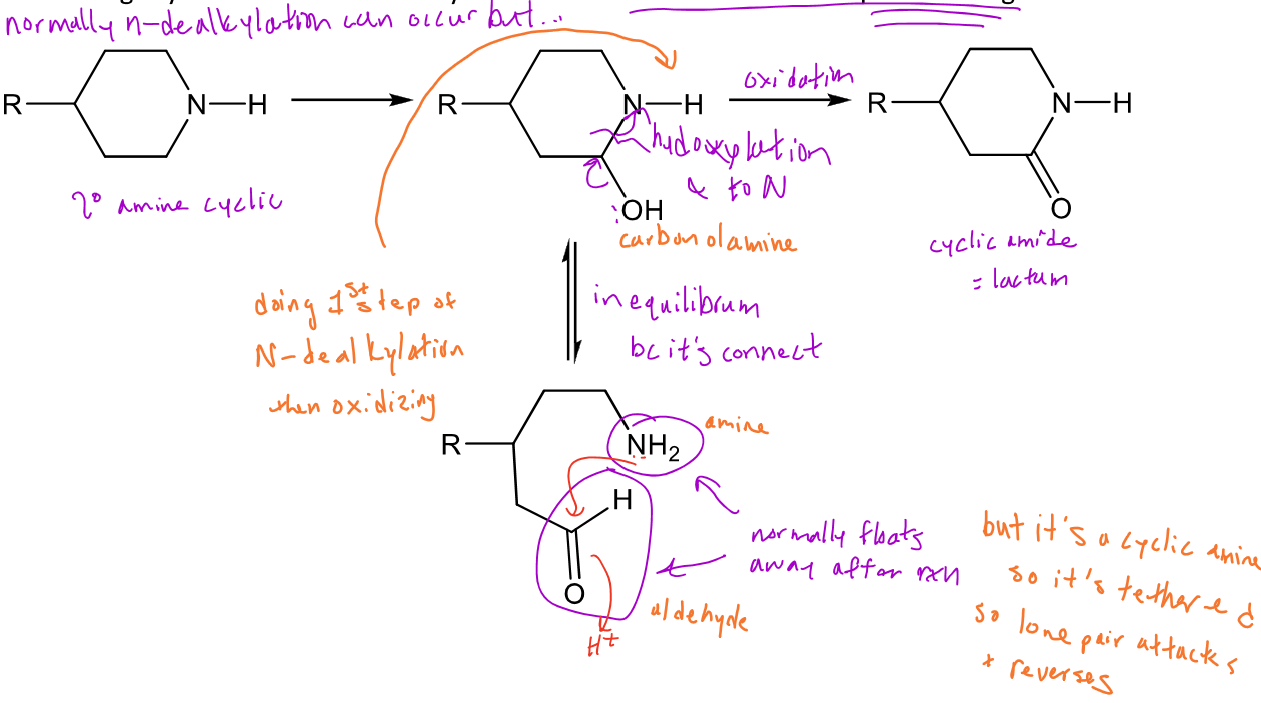
N-dealkylation of a cyclic amine
Cyclic amines can still undergo N-dealkylation with hydroxylation followed by fragmentation to break the ring
-Because they're in a ring system, what can also happen is that the hydroxylated ring can undergo further oxidation, with the nitrogen attacking the carbonyl to form a cyclic amide
N-dealkylation of amides
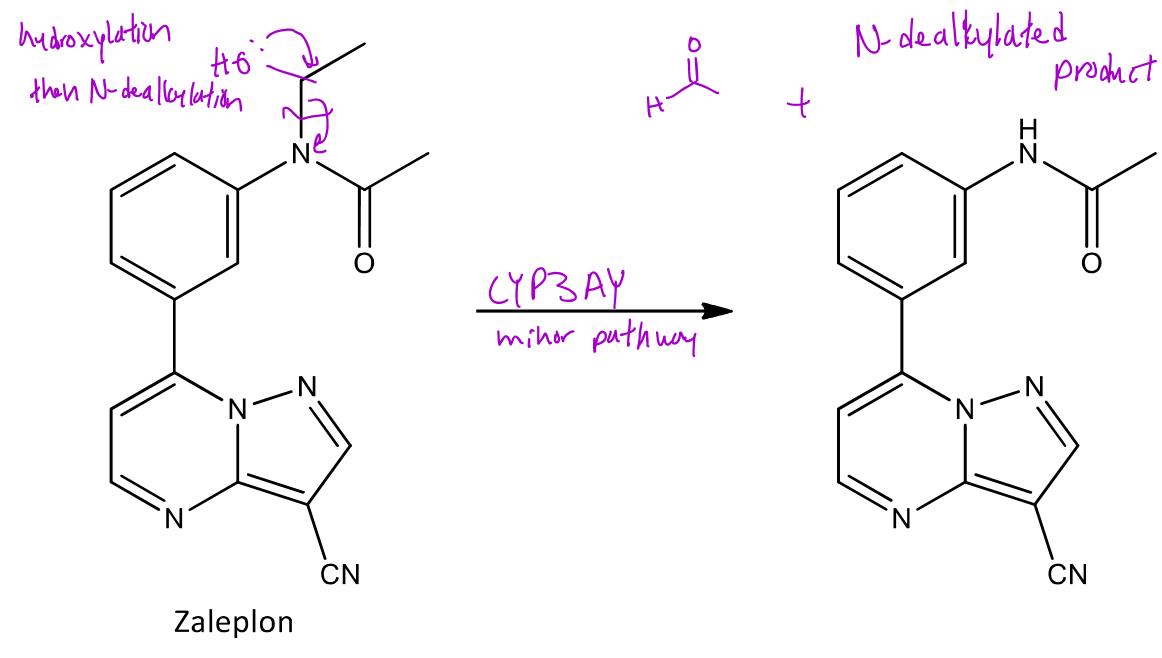
Amides can also undergo N-dealkylation where the α carbon to the nitrogen of the amide will be hydroxylated and then fragmented off which will form an aldehyde and a new amide
Ex. Zaleplon will undergo N-dealkylation at the amine which which form formaldehyde and a new version of the amide
N-oxidation
A type of reaction in phase 1 metabolism that can hydroxylate the nitrogen of any degree amine
-In 1° amines, the amine will become an hydroxylamine which can be further oxidized into a nitroso group
-The nitroso group can also be spontaneously rearranged into an oxime
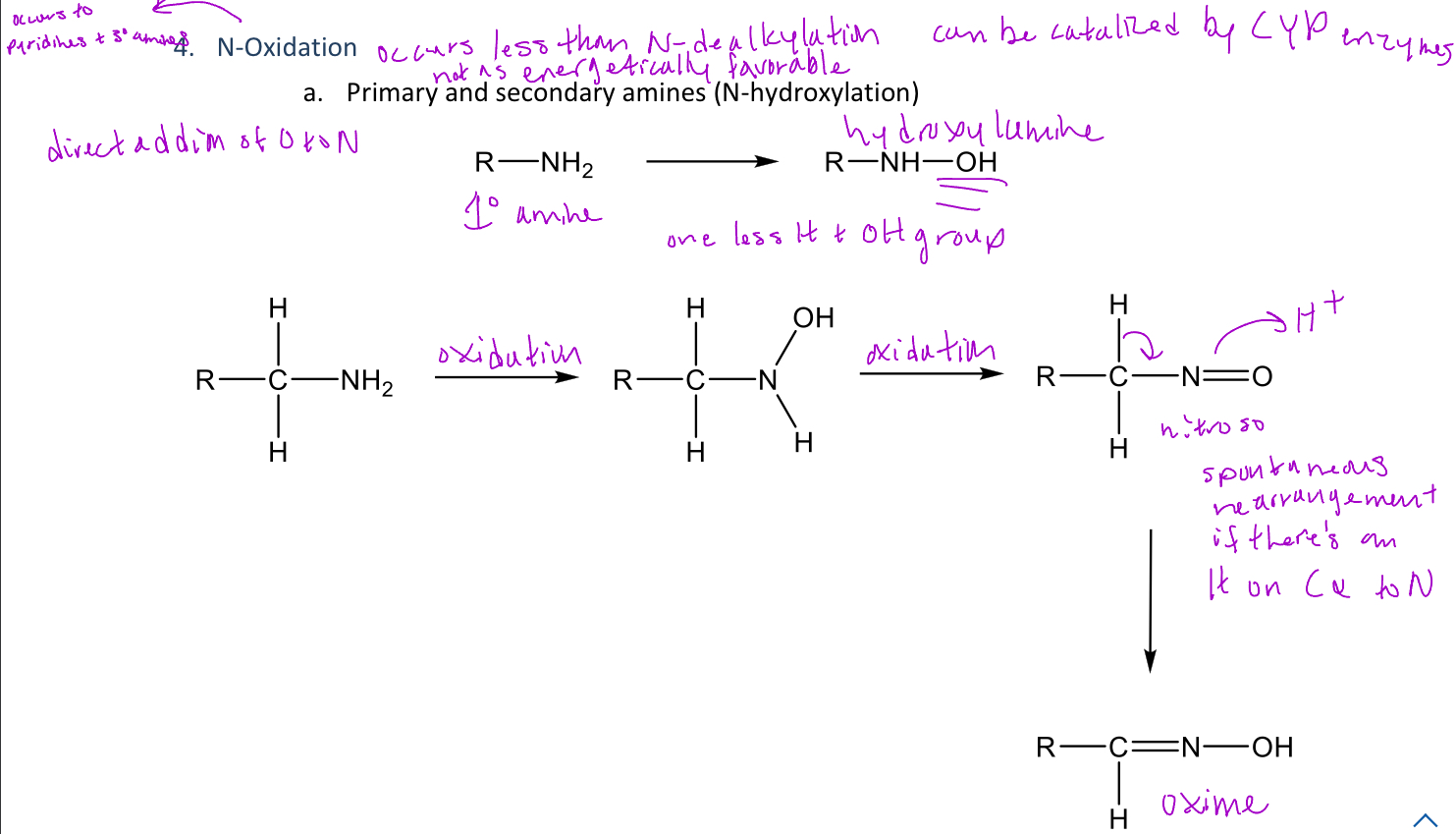
Oxime
A functional group that can be spontaneously formed from a nitroso group of 1° amine N-oxidation
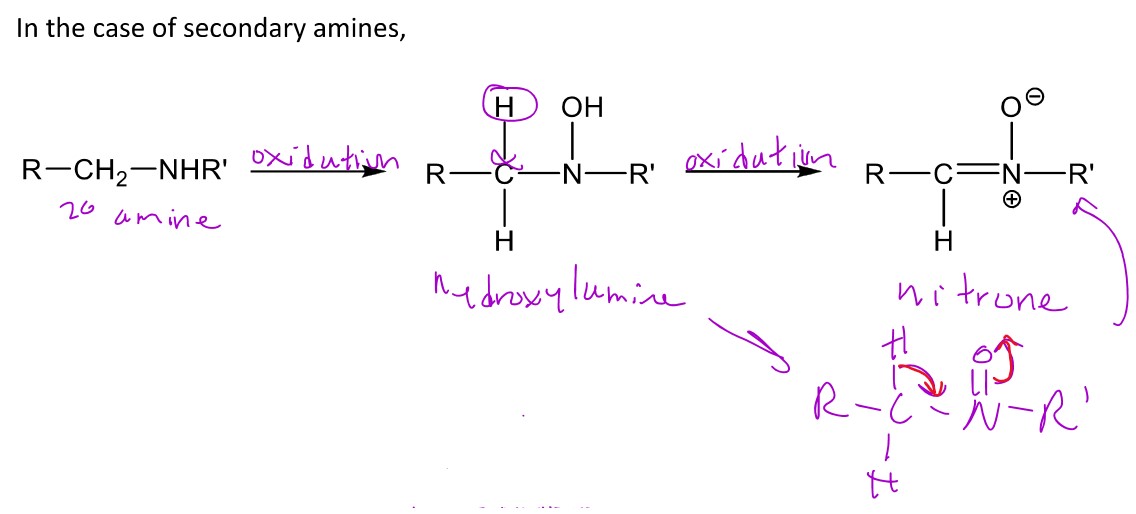
N-oxidation of a 2° amine
When a 2° amine undergoes N-oxidation, it can form the hydroxylamine which can further be oxidized into the nitroso or it can be converted into a nitrone
N-oxidation of a 3° amine/pyridine
When a 3° amine undergoes N-oxidation, an N-oxide will form which puts a (+) charge on the nitrogen and a (-) charge on the oxygen
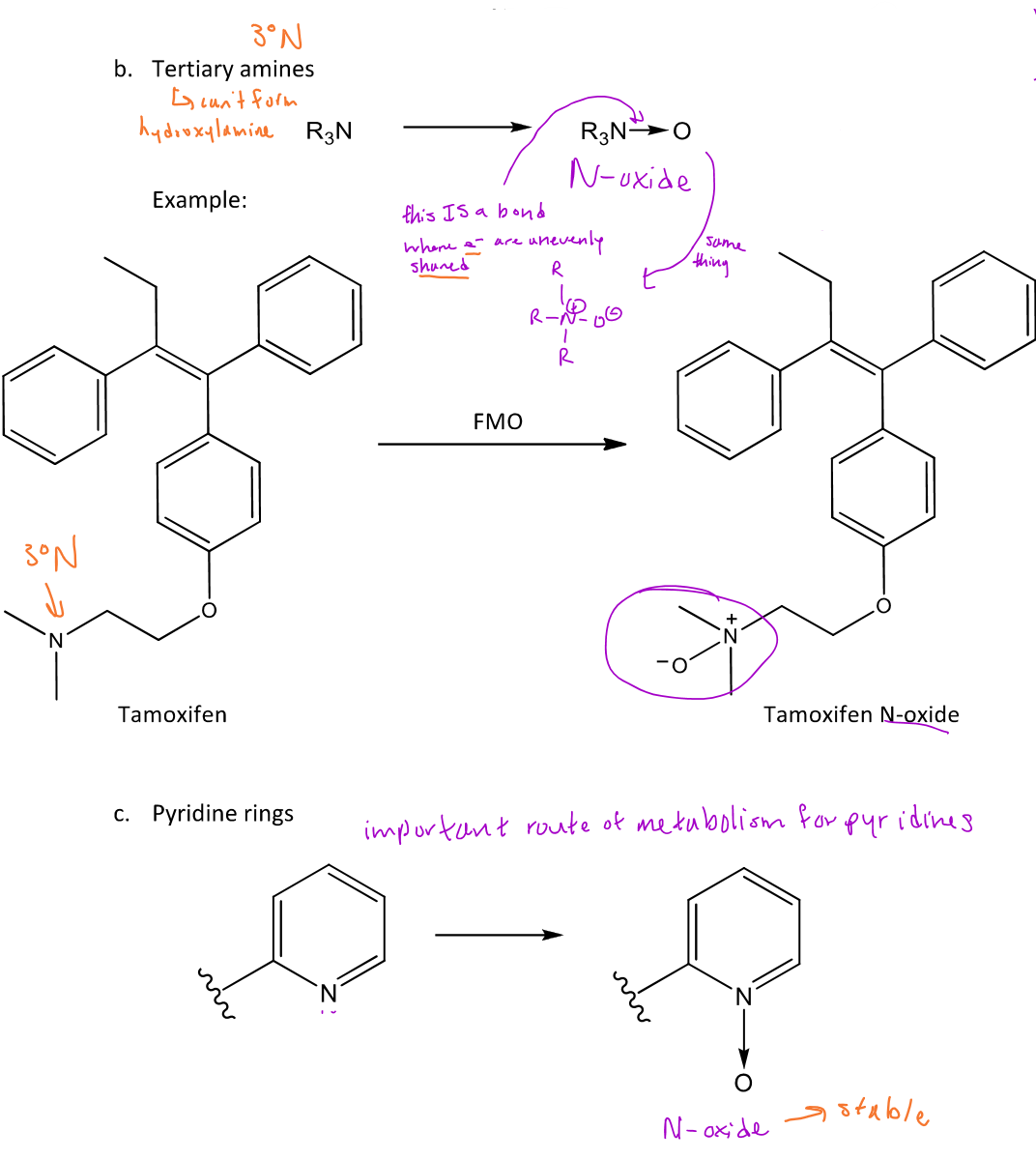
N-oxidation of amides
N-oxidation of amides can also occur usually when the amide is bound to an aryl group
-This reaction will add an OH group to the nitrogen of the amide
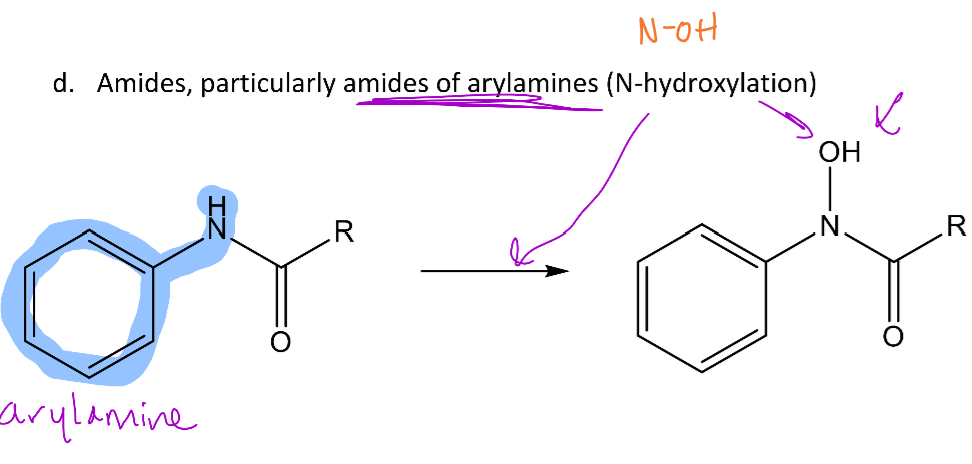
Desulfurization
A type of reaction in phase 1 metabolism that converts the sulfur of a thiocarbonyl into a carbonyl with an oxygen
Ex. thiopental contains a thiocarbonyl and when it undergoes desulfurization, the sulfur is converted to an oxygen (which is shown in the image as the middle carbonyl)
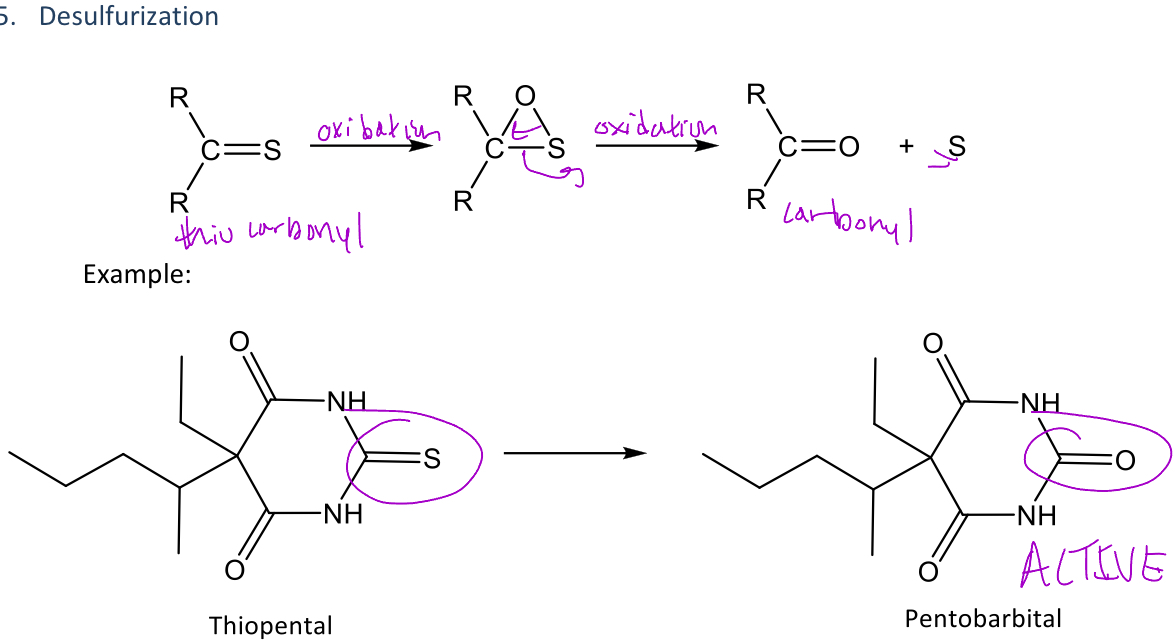
Sulfoxidation
A type of reaction in phase 1 metabolism that is seen in thioethers and thiol compounds
-In thioethers (sulfides) the functional group is converted into a sulfoxide (ketone with sulfur instead of oxygen)
-In thiol compounds (-SH), the thiol is oxidized into sulfenic acid which will react with another thiol compound to produce a disulfide bridge
2. Arylnitro groups
3. Carbonyls
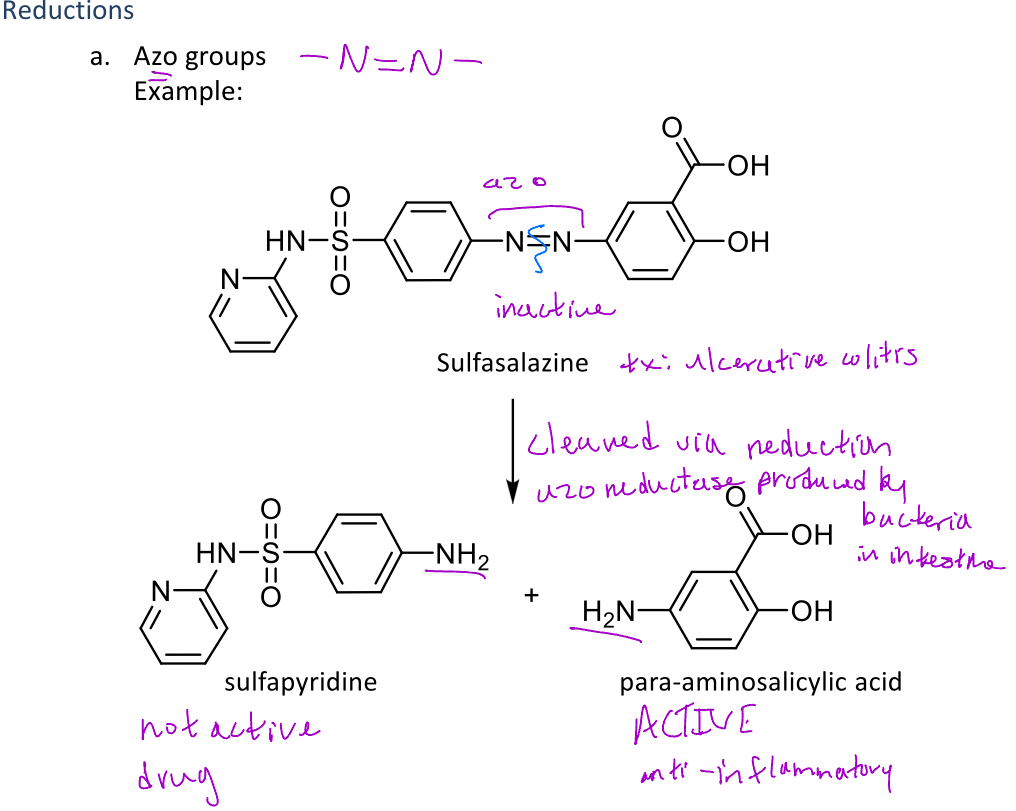
Azo group reduction
A type of reduction reaction that occurs in phase 1 metabolism that cleaves an azo group
Ex. Sulfasalazine contains an azo group that is cleaved via reduction with azo reductase enzymes which creates sulfapyridine and para-aminosalicylic acid (active component)
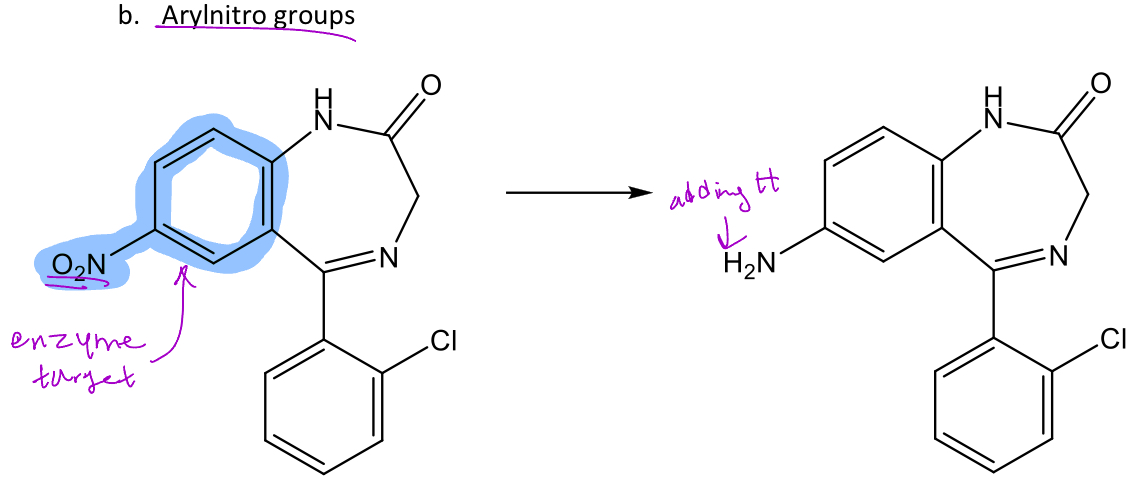
Arylnitro group reduction
Arylnitro is a nitro group bound to an aromatic ring -These would undergo reduction in phase 1 metabolism to form amines Ex. Clonazepam (in image) is converted from an aryl nitro to an arylamine
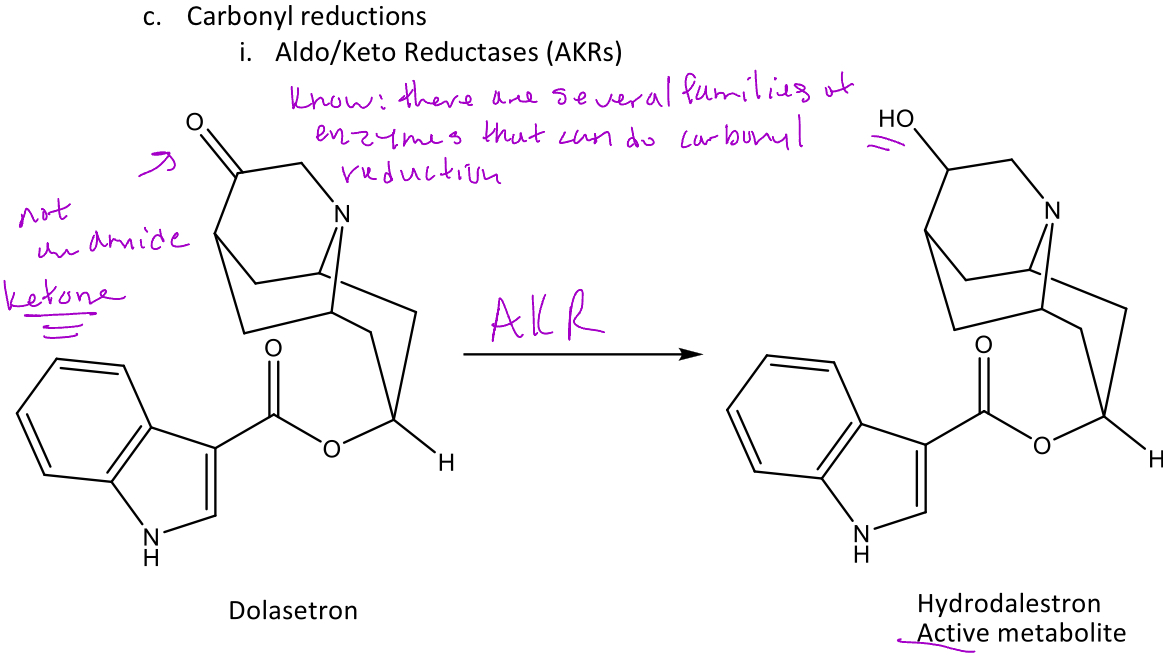
Carbonyl reductions
A group of reactions in phase 1 metabolism involving the reduction of carbonyls (aldehydes and ketones) into alcohols
Ex. dolasetron will undergo a carbonyl reduction with aldo/keto reductase (AKR) which converts the ketone into an alcohol
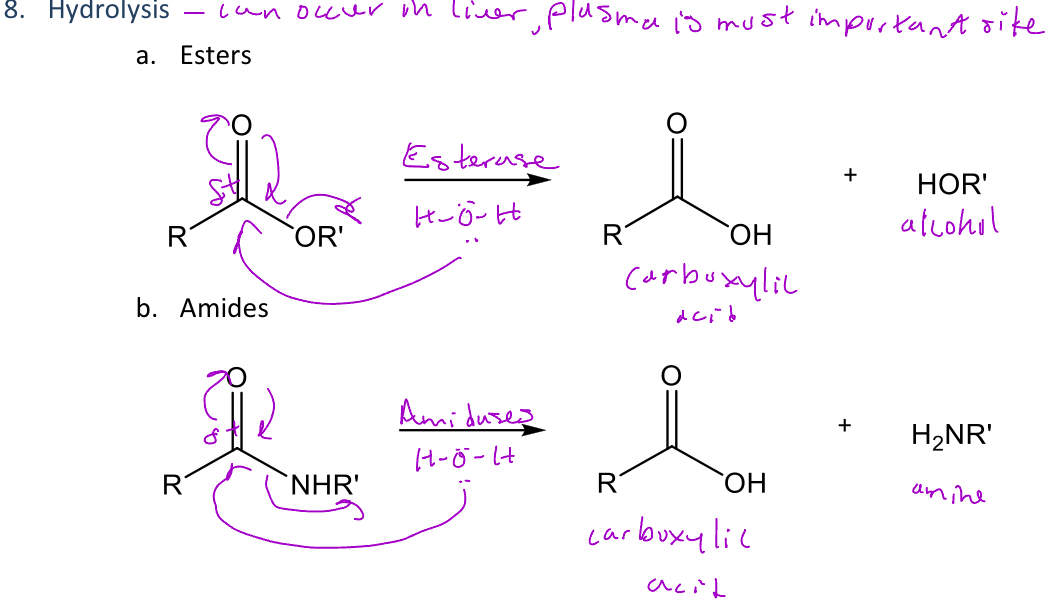
Hydrolysis
A type of reaction in phase 1 metabolism that *breaks down esters or amides into a carboxylic acid and an alcohol or amine, respectively
-When it's an ester, an esterase is used to separate the functional group
-When it's an amide, an amidase is used to separate the functional group
Rate of hydrolysis depending on the functional group
Esters > amides in the rate of hydrolysis because the ester has two highly electronegative atoms that pull electrons away from the C of the carbonyl (which makes it very electrophilic)
-There are two types of amides depending on what the nitrogen is bound to:
-If the nitrogen of an amide is also part of an arylamine, the aryl group is electron withdrawing but is also electron donating towards the C of the carbonyl which is why it has a slower rate of hydrolysis than the ester
-If the nitrogen of the amide is connected to an alkyl group, the alkyl group is more electron donating towards the nitrogen which makes the carbonyl less electrophilic
2. Alcohol and aldehyde oxidations
3. Purine oxidations
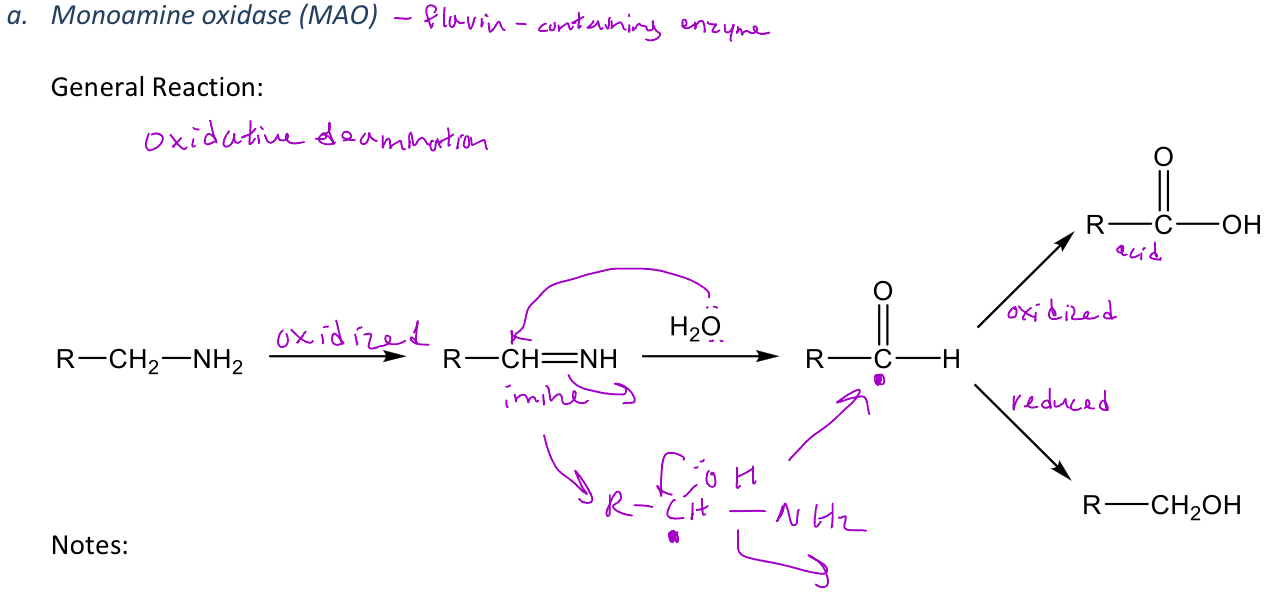
Monoamine oxidase (MAO) metabolism
A miscellaneous reaction in phase 1 metabolism in which monoamine oxidase (MAO) only works when the amine only has methyl groups added to it
-MAO will convert amines into carboxylic acids or alcohols
-The carbon that's alpha to the nitrogen cannot have a CH3 group on it (which is why amphetamine isn't broken down by MAO)
Alcohol and aldehyde oxidations
A miscellaneous reaction in phase 1 metabolism in which an alcohol can be converted to an aldehyde via alcohol dehydrogenase (ADH)
-This aldehyde can further be oxidized into a carboxylic acid via aldehyde dehydrogenase (ALDH)
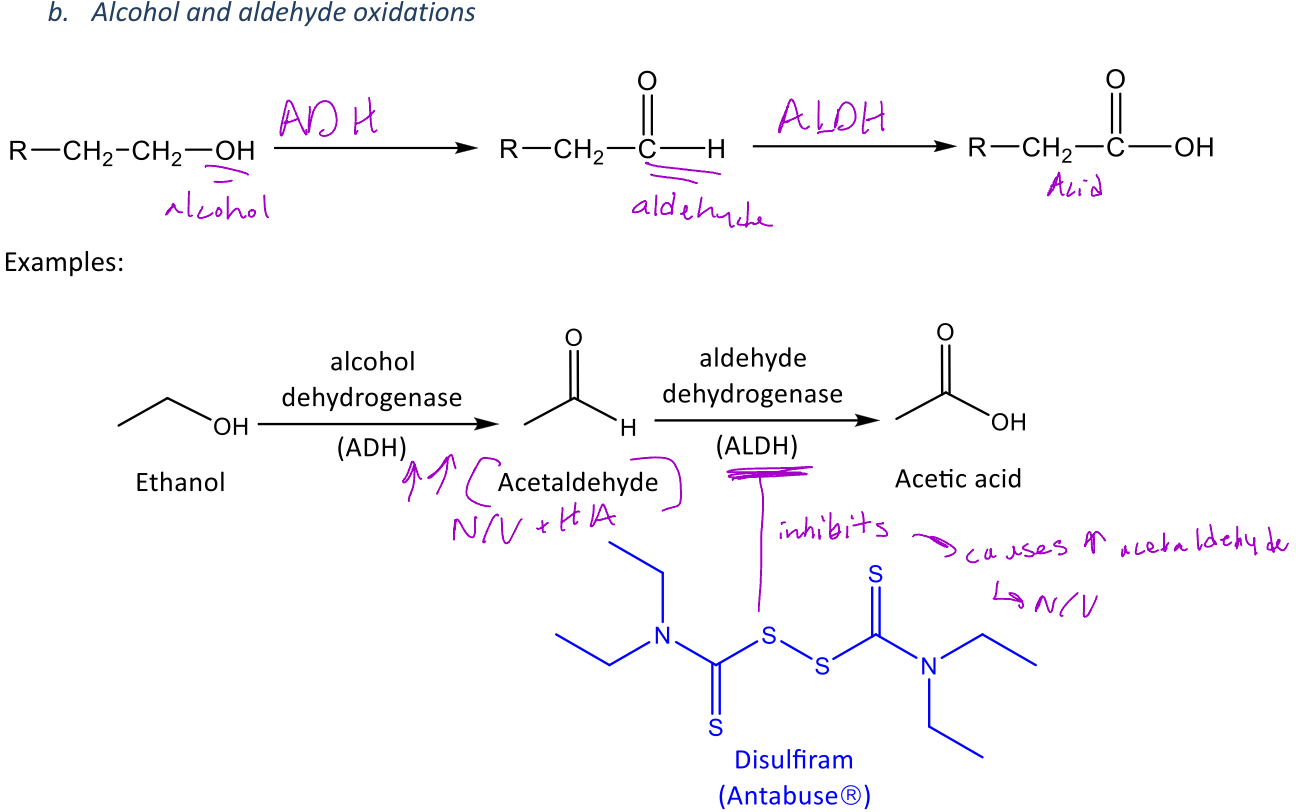
Metabolism of ethanol
Ethanol is converted into acetaldehyde via alcohol dehydrogenase (ADH)
Acetaldehyde is converted to acetic acid via *aldehyde dehydrogenase (ALDH)
-Acetaldehyde is significant because it will cause N/V and HA at high concentrations
Disulfiram
A type of drug used use treat alcoholism which inhibits ALDH
-This causes an increase in acetaldehyde when alcohol is drank which induces vomiting
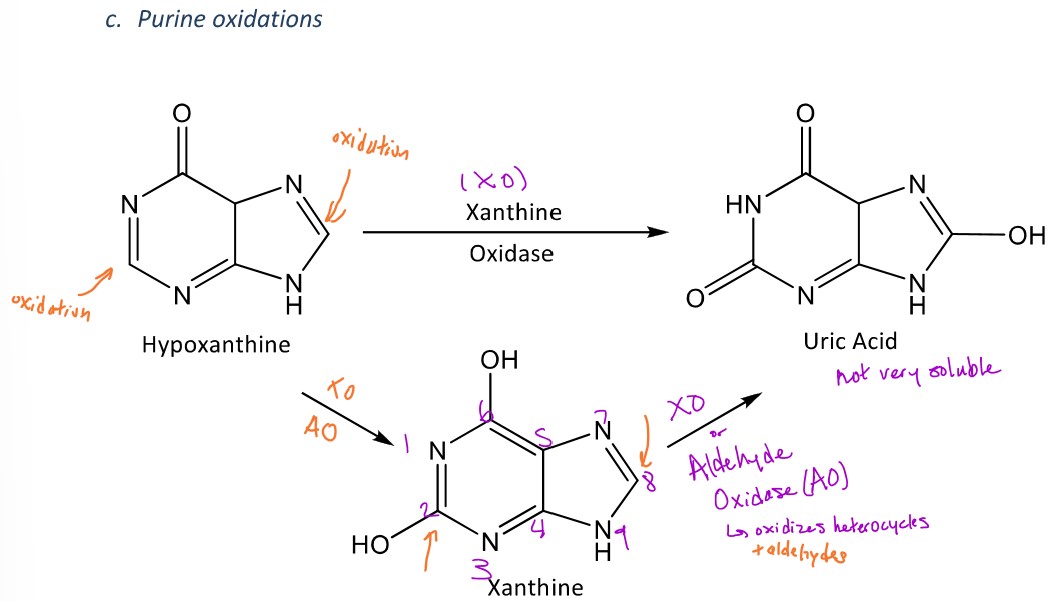
Purine oxidation
A miscellaneous reaction in phase 1 metabolism in which a purine ring will be oxidized
Ex. Hypoxanthine (in image) will be oxidized into xanthine by adding an OH group to the C2 position and then xanthine oxidase will oxidize xanthine into uric acid by adding an OH group at the 8 carbon within the bicyclic ring which will form uric acid
-Aldehyde oxidase (AO) can also form uric acid from xanthine because AO oxidizes heterocycles (like purine) and they like oxidizing groups at C2 (hydroxyl or amino groups)