Tumour Targeting Strategies
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
General Cancer treatments refresher
Surgery - best option (usually followed up with radiotherapy, chemotherapy or both)
Radiotherapy
Systemic chemotherapy
Targeted therapy
What are the types of surgery and briefly describe their purpose?
- Curative surgery removes the cancerous tumour or growth from the body. Surgeons use curative surgery when the cancerous tumour is localized to a specific area of the body
-Preventive surgery is used to remove tissue that does not contain cancerous cells, but may develop into a malignant tumour
-Debulking surgery removes a portion, though not all, of a cancerous tumor. It is used in certain situations when removing an entire tumor may cause damage to an organ or the body. e.g. brain tumour
-Palliative surgery is used to treat cancer at advanced stages. It does not work to cure cancer, but to relieve discomfort or to correct other problems cancer or cancer treatment may have created.
What are the types of radiotherapy?
-We want to spare normal tissues so we want the radiotherapy to be as localised as possible
External beam (high energy Xrays): a medical device known as a linear accelerator (or LINAC) generates ionising radiation (IR). The radiation beam is targeted to the area where the tumour is located. High doses of IR cause cancer cell death by inducing irreparable DNA damage.
Proton beam (high energy protons): The proton beam can be more accurately targeted so that more healthy tissue can be spared
Internal radiotherapy (brachytherapy and systemic therapy): A radiation source is placed inside the body. The source is solid (seeds, ribbons, capsules) in the case of brachytherapy, or liquid in the case of systemic therapy
Detailed MOA of Vinca alkaloids?
Think chromosome arrangement
- During metaphase (mitosis), a mitotic spindle formsterm-0
- All chromosomes align in middle
- In anaphase, chromosomes segregate and migrate along microtubules until they reach spindle poles where daughter cells separate each with right amount of genetic info
- Alkaloids bind to tubulin preventing assembly of microtubule bundles and thereby prevent assembly of mitotic spindle so DNA cannot segregate and migrate to poles
- Spindle breaks apart and cell dies due to damage
What are the types of chemotherapy drugs available and describe their basic MOA's
- Alkylating agents: act directly on DNA = cross-linking, abnormal base pairing, DNA strand breakage = these agents have the greatest value in treating slow-growing tumours
- Antitumour antibiotic: bind to DNA and prevent RNA synthesis e.g., Doxorubicin, mitoxantrone and bleomycin
- Vinca alkaloids: interfere with cell division—target tubulin and disrupt the mitotic spindle—there are five major alkaloids in use: Vinblastine, Vinorelbine, Vincristine, Vindesine and Vinflunine
-Antimetabolites: disrupt nucleic acid synthesis by interfering with production of a major nucleotide metabolites or by substituting for the natural metabolite—include folic acid antagonists like methotrexate, purine antimetabolites such as 6-mercaptopurine / 6- thioguanine, fludarabine phosphate, pentostatin and cladribine; pyrimidine antimetabolites such as 5-fluorouracil, 5-fluorodeoxyuridine, cytarabine and gemcitabine
What are the different examples of vinca alkaloids?
vinblastine (VBL), vincristine (VCR), vinorelbine (VRL), vindesine (VDS) and VFN
Uses of Vinca alkaloids (from the 5 different types) in oncology and common side effects?
just have a couple of examples for each type
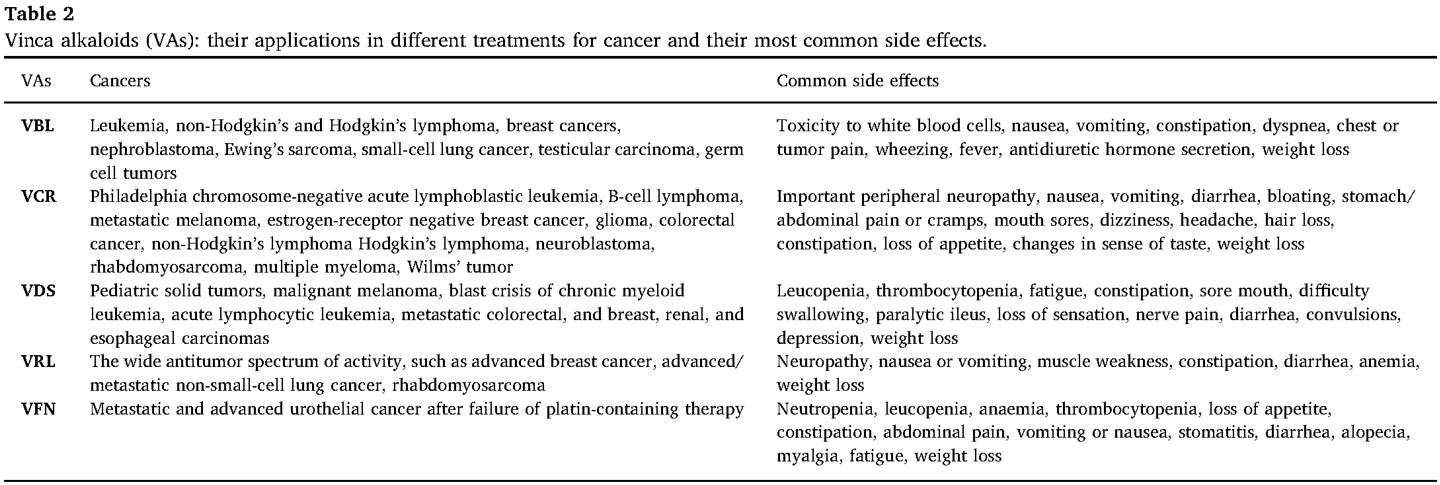
What is the mechanism of action of antimetabolites?
-They mimic natural nucleotides, interfering with DNA/RNA synthesis but cells can't use these to synthesise DNA/RNA
Example:
-Methotrexate inhibits dihydrofolate reductase (DHFR), blocking purine and thymidylate synthesis (DNA building blocks) =prevents generation of tetrahydrofolic acid
-5-Fluorouracil (5-FU) inhibits thymidylate synthase, preventing DNA replication
Note: Nucleotide is a nucleoside with a P added
Define Genotype
the genetic makeup of an individual organism and can also refer to the specific gene variants (alleles) carried by an organism
Define Phenotype
the observable physical properties of an organism which are determined by its genotype and influenced by its environments
Define Molecular profiling:
the generation of a biological specimen's blueprint based on their molecular phenotype e.g., the whole genome sequence of a tumour sample
Define Biomarker
: a defined characteristic that is measured as an indicator of normal biological processes, pathogenic processes or responses to an exposure or intervention
Define an oncongene
a mutated gene, derived from a normal cell growth-regulating gene called a proto-oncogene
What do we need to consider to provide targeted therapy in a patient to target a tumour?
What is precision medicine ?
-Cancer research for genes overexpressed/mutated in cancer cells
-Molecular profiling of tumour to Characterise the tumour, through surgery and biopsy
—
-Precision medicine is A medical model that proposes the customisation of healthcare with medical decisions, treatments, practices
-Refers to a specific feature of a tumour to use to drive the choice of treatment
-As we learn more about the molecular determinants of these tumours, the genotype of these tumours dictates to a large extent how they grow and how they respond to treatment—this is key as it necessitates that ach patient needs to be treated differently
—
Regarding cancer:
- Mutations lead to function and shape change of proteins
- Cancer cells restructure in a way where signals change
- Good therapy is something that
-1) changes this induced structure causing it to collapse = a single blow destroying entire cell
- 2) same move should cause no harm to normal cells
What is the difference between molecular vs cellular phenotyping of cancer cells
Cellular phenotyping:
-Viable (here cancer) cells are treated with genetic manipulation which we suspect is the driver of the cancer
-We can then look at the cells under the microscope = allows us to see effects
-physical/functional manifestations of cancer cells
Molecular Phenotype
Refers to the biochemical and genetic characteristics of cancer cells at the molecular level
-Genetic changes
-Signalling pathways
-Transcriptional profiles
-Protein overexpressiono
-Measured via genomic sequencing
-Governs cellular decision-making
What are the hallmarks of cancer in precision medicine (9 of them)? What is the most important?
Targeted therapies of them all? - discussed in lecture 2
Most important:
-Sustaining proliferation signaling (focus of lecture one)
Key Hallmarks of Cancer & Targeted Therapies – Simplified Notes
1️⃣ Sustaining Proliferative Signalling
Cancer cells constantly receive growth signals due to oncogenic kinases.
Inhibitors of these kinases block excessive growth signals: EGFR inhibitors: Erlotinib, Afatinib, Osimertinib. BRAF inhibitors: Vemurafenib, Dabrafenib. Bcr-Abl inhibitors: Imatinib, Dasatinib, Asciminib (used in CML).
2️⃣ Evading Growth Suppressors:
Cyclin-dependent kinases (CDKs) control cell cycle progression.
The Retinoblastoma (RB) gene (a tumour supressor gene) normally restricts CDK activity to prevent uncontrolled cell growth.
Loss of RB function leads to constant CDK activation → uncontrolled tumour growth.
CDK4/6 inhibitors help suppress tumour growth when RB function is impaired: Palbociclib, Ribociclib, Abemaciclib.
3️⃣ Avoiding Immune Destruction
Cancer cells escape immune attack by using immune checkpoints (PD-1, PD-L1, CTLA-4) to turn off T-cells.
Checkpoint inhibitors restore immune function: PD-1 inhibitors: Pembrolizumab, Nivolumab.
PD-L1 inhibitors: Atezolizumab.
CTLA-4 inhibitor: Ipilimumab.
4️⃣ Tumour-Promoting Inflammation
Chronic inflammation creates a tumour-friendly environment for growth and mutation.
Celecoxib (a COX-2 inhibitor, NSAID) helps reduce inflammation and is used for patients with Familial Adenomatous Polyposis (FAP), a cancer-predisposing condition.
5️⃣ Inducing Angiogenesis (New Blood Vessel Formation)
Tumours need new blood vessels to grow and spread (metastasis).
VEGF (Vascular endothelial growth factor) inhibitors like Bevacizumab stop angiogenesis.
Successful therapies must also evade immune detection to remain effective.
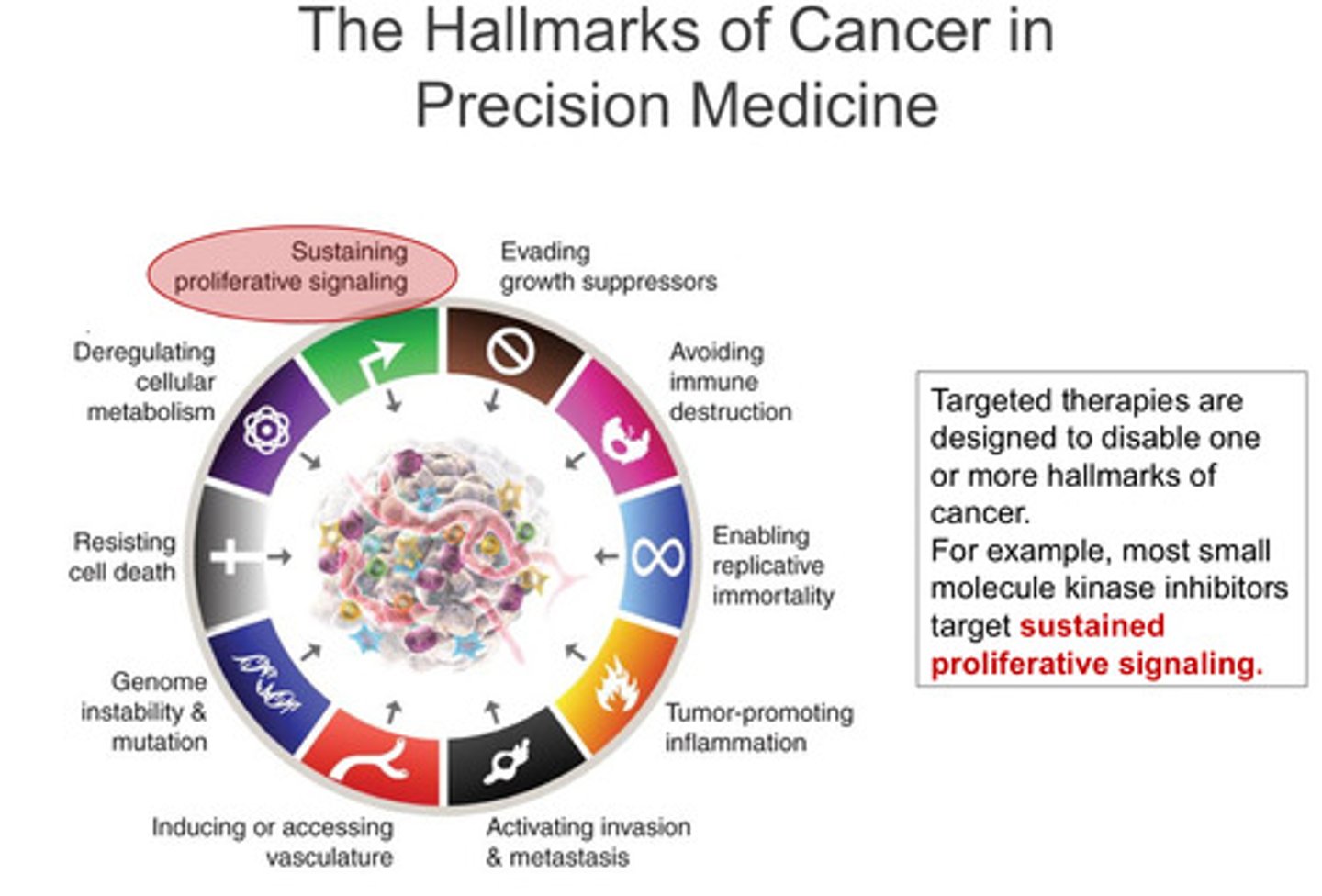
What are the hallmarks of cancer in precision medicine (9 of them)? What is the most important?
Targeted therapies of them all? - discussed in lecture 2 (PART 2)
Note: there is nothing missing don't worry
6️⃣ Targeting Genome Instability & Mutation
Some tumours have defective DNA repair genes (e.g., BRCA1/2), leading to genomic instability.
PARP inhibitors specifically target these tumour cells, making it difficult for them to repair DNA damage.
7️⃣ Resisting Cell Death (Apoptosis Evasion)
Cancer cells avoid apoptosis by overexpressing anti-apoptotic proteins (e.g., BCL-2).
Venetoclax (a BH3 mimetic) inhibits BCL-2, restoring apoptosis and is used for certain types of leukaemia.
Why do targeted therapies work?
-Cancer cells have many mutations that alter the normal function of genes and the proteins they encode
-Only some mutant proteins will be critical for the survival of tumour cells
-These mutant proteins create an unusual cancer-specific dependence that can be therapeutically exploited (oncogene addiction
What makes a good cancer drug in reference to oncogene addiction?
A good cancer drug will selectively kill a cancer cell that is addicted to the drug target (the ultimate goal of therapy should be to shrink tumours, not just stop them from growing)
What is Oncogene Addiction: the basis of targeted therapies?
- Targeted therapies: inactivate cancer-causing mutant proteins
- These cancer causing mutations part of the tumour's genotype
- These consequences of inhibiting cancer-causing genes constitute the phenotypes of treatment
(Two ideal results, differentiated cell or apoptosis )
Oncogene addiction= Dependence of a specific type of cancer cells on a specific oncogene
Example of targeted therapy: What is Chronic myeloid leukaemia (CML), what is its unique chromosome abnormality and what is the driving force for it to be become malignant?
-An example of targeted therapy
- CML: proliferative disorder of haematopoietic stem cells
- Philadelphia Ph chromosome (a reciprocal translocation between chromosomes 9 and 22.) = unique chromosomal abnormality which creates a fusion protein called bcr-abl - causes uncontrolled cell proliferation in CML
- Bcr-Abl tyrosine kinase: a single molecular abnormality that causes transformation of a haematopoietic progenitor into a malignant clone - leukemogenic
How does Imatinib (Gleevec) treat CML?
- Imatinib mesylate is a selective inhibitor of Bcr-Alb kinase
- Inhibition of this kinase is effective therapy for CML
- Imatinib mesylate is antileukemic
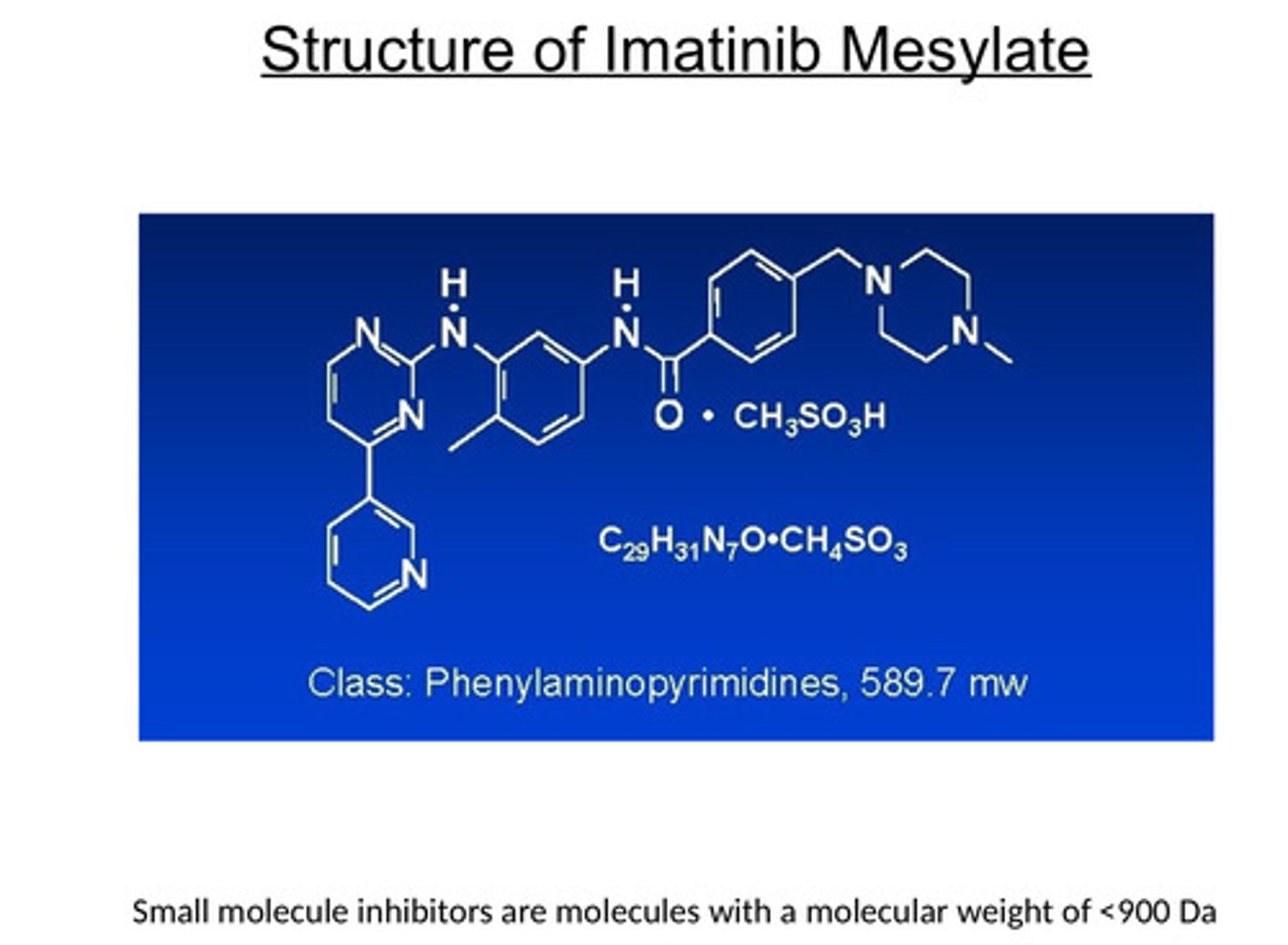
What type of inhibition do we use for cancers inhibiting kinases?
Competitive inhibition
Has 1) kinase domain and 2) substrate domain
-Drug will bind to the substrate domain = endogenous substrate can't bind = can't carry out its function
Define an oncogene
a mutated gene that has the potential to cause cancer. Before an oncogene becomes mutated, it is called a proto-oncogene, and it plays a role in regulating normal cell division
Why is the oncogene RAS not more targeted?
-difficult to make direct inhibitors of RAS
-only one direct KRAS inhibitor - sotorasib
-Approved for the treatment of RasG12C+ lung cancer
What is the importance of biomarkers?
-they can help identify patients most likely to respond to a specific targeted therapy
-This is why molecular diagnostic tests are nowadays generally co-developed with therapeutic agents (companion diagnostics)
Why are Targeted therapies are short-lived and not curative?
- They do stop working after some time
- With targeted therapies we understand the biology of the mutant gene
- Can also understand the mechanisms behind resistance
- Mutations block drug ability to bind and inhibit target
-Activation of alternative survival pathways
e.g. EGFR as the target - Second site mutations in EGFR drive resistance to 1st generation EGFR tyrosine kinase inhibitors (TKIs) - note: First-generation EGFR inhibitors (e.g., Erlotinib) blocked EGFR but resistance developed.
-T790M mutation caused resistance.
-Osimertinib (third-generation EGFR inhibitor) specifically targets T790M mutants
Why are kinase inhibitors an important class of targeted therapies?
Kinases regulate cell signalling and are frequently mutated in cancer.
Blocking cancer-specific kinases halts tumour growth.
Example:
Imatinib (Bcr-Abl inhibitor for CML)
Osimertinib (EGFR inhibitor for lung cancer)
Why is tumour profiling essential in modern oncology?
-Identifies mutations driving cancer.
-Matches patients with the most effective targeted therapy.
-Reduces unnecessary toxicity.
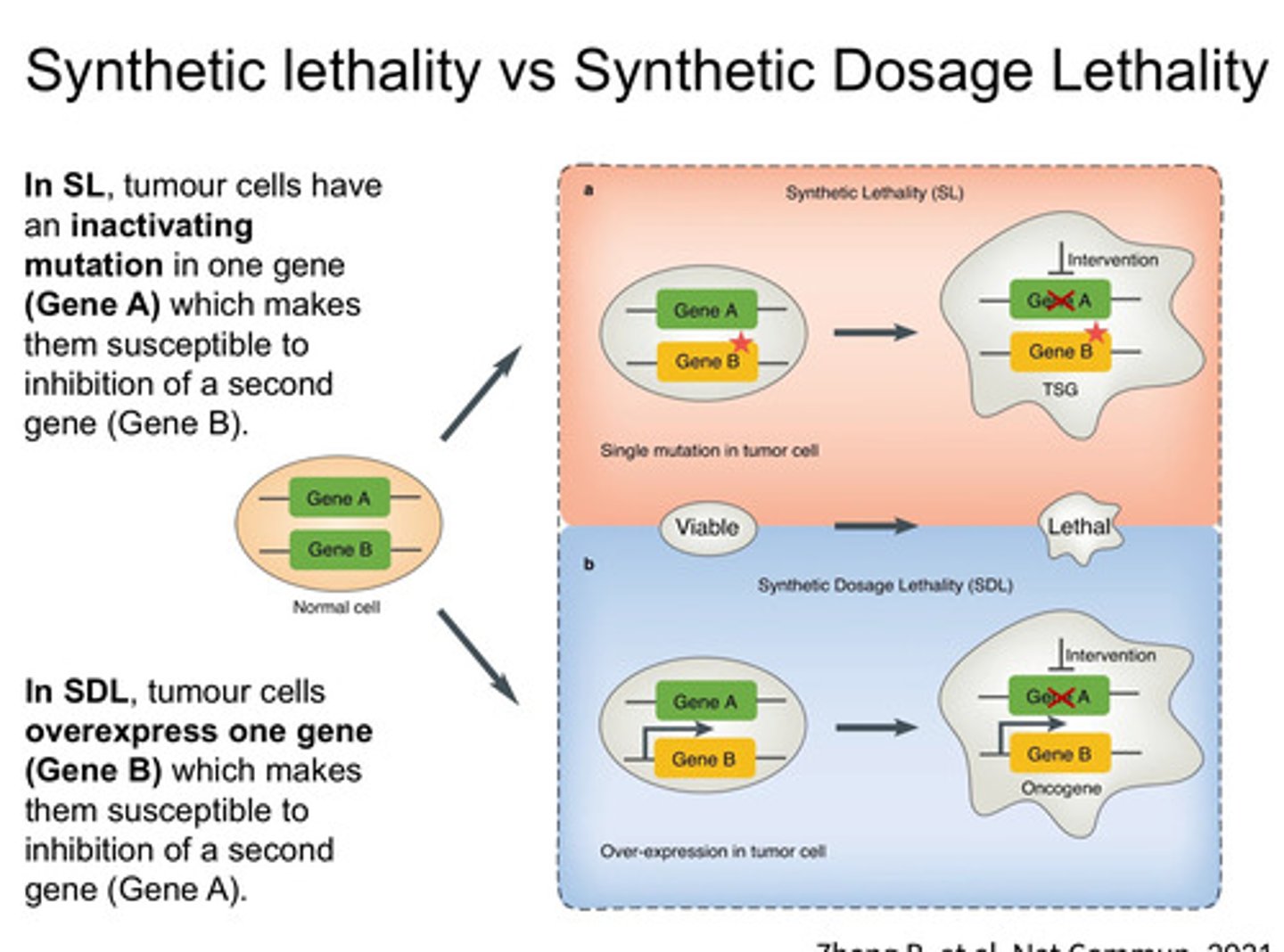
What is Synthetic Lethality vs Synthetic Dosage Lethality
-Synthetic Lethality – When two mutations together cause cell death, but individually, they don’t (e.g., PARP inhibitors + BRCA mutations - BRCA-mutated cancers rely on PARP1 for DNA repair; PARP inhibitors (e.g., Olaparib) induce lethal DNA damage in these cells).
-Targets loss-of-function mutations
-Synthetic Dosage Lethality – When overexpression of one gene makes cells hypersensitive to inhibition of another gene, leading to cancer cell death.
-Targets gain-of-function mutations
-Exploits dependencies created by oncogene overexpression.
-E.g. Overexpression of MAD2 (a spindle checkpoint gene) sensitizes cancer cells to inhibition of PP2A (a phosphatase)
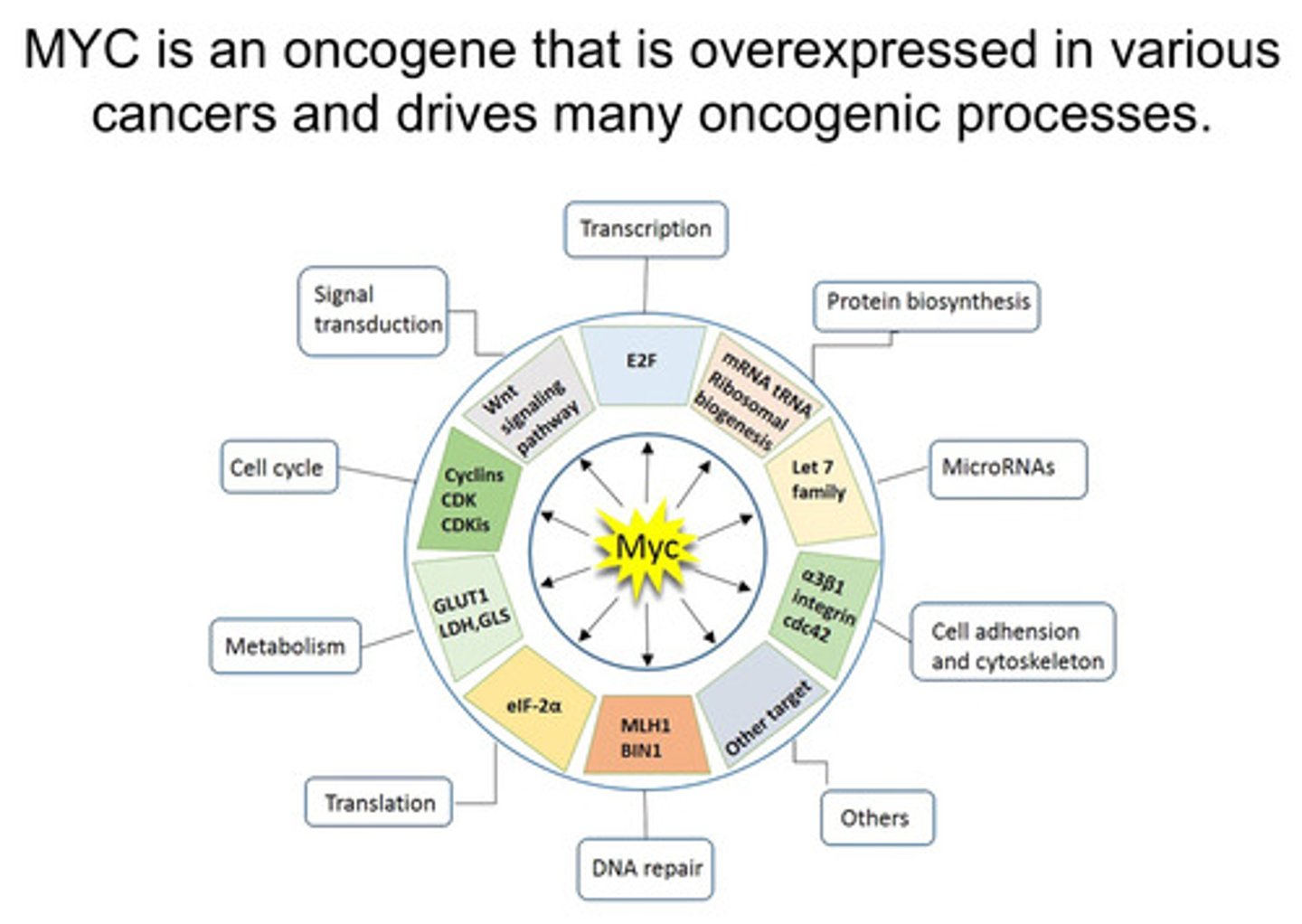
What oncogenenic processes does MYC drive?
Describe SDL using MYC as the example
MYC example of SDL:
-e.g. SDL occurs when overexpression of MYC (an oncogene) creates a dependency on another gene/protein, and inhibition of that partner becomes lethal to cancer cells
- MYC is an oncogene that is overexpressed in various cancers and drives many oncogenic processes
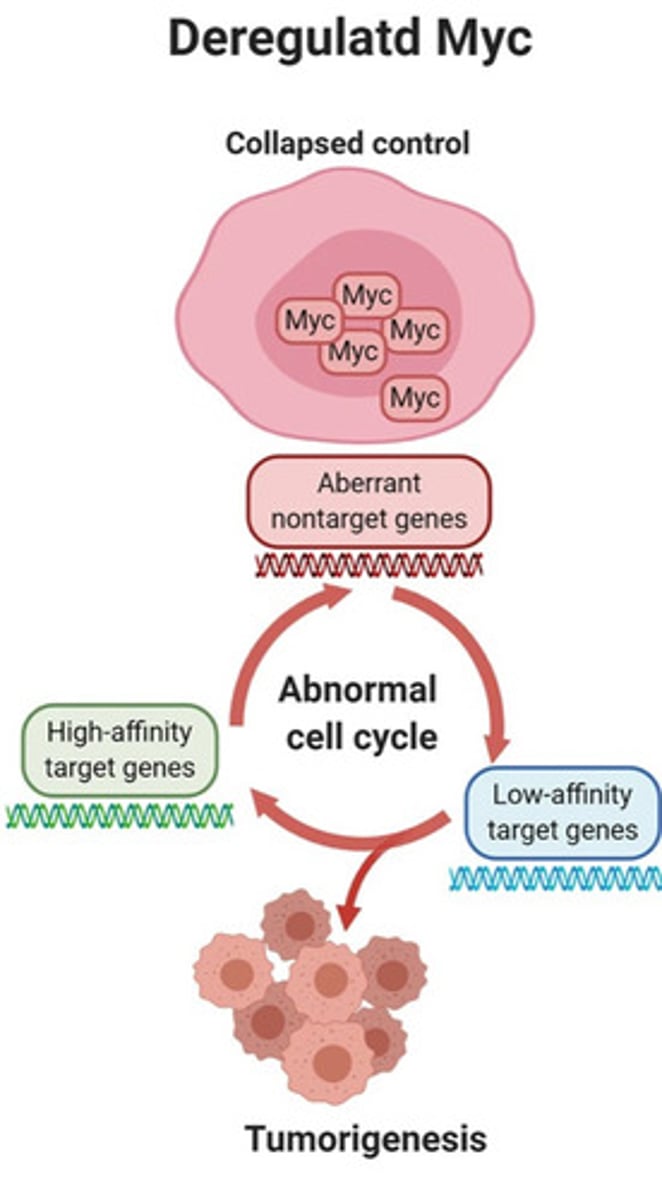
- Overexpression of MYC causes abnormal expression of cancer-promoting genes (tumorigeneisis)
- MYC-driven overexpression of cancer-promoting genes generates tumour-specific liabilities e.g. For example, Aurora A is a kinase whose expression is significantly increased by aberrant activation (the unregulated or inappropriate activation of a gene) of MYC. Aurora A inhibitors can inhibit the growth of MYC amplified tumours in pre-clinical models
What is lineage dependence in regards to tumour targeted therapy?
-Lineage dependence describes how tumors retain molecular dependencies on genes essential for their original tissue's development and function.
These genes often encode:
-Lineage-survival oncogenes
-Lineage-specific transcription factors
-Developmental signaling pathways
-Targeting lineage dependencies allows precise cancer treatment with fewer side effects. e.g. MYC-driven cancers experience high replication stress → Blocking PRKDC (DNA repair gene) selectively kills them.
Why is This Important for Cancer Treatment?
✅ Precision Medicine – Tumours retain vulnerabilities related to their original tissue, which can be targeted for treatment.
✅ Better Drug Targeting – Unlike traditional chemotherapy, lineage-targeted therapies are more precise.
✅ Lower Resistance Risk – Lineage dependencies are less likely to develop drug resistance compared to oncogene-targeted therapies.
Challenges:
-Some lineage genes (e.g., MITF) are hard to target with drugs.
-Some normal tissues also express these genes, leading to potential side effects.
-Not all tumours from the same lineage share the same dependency.
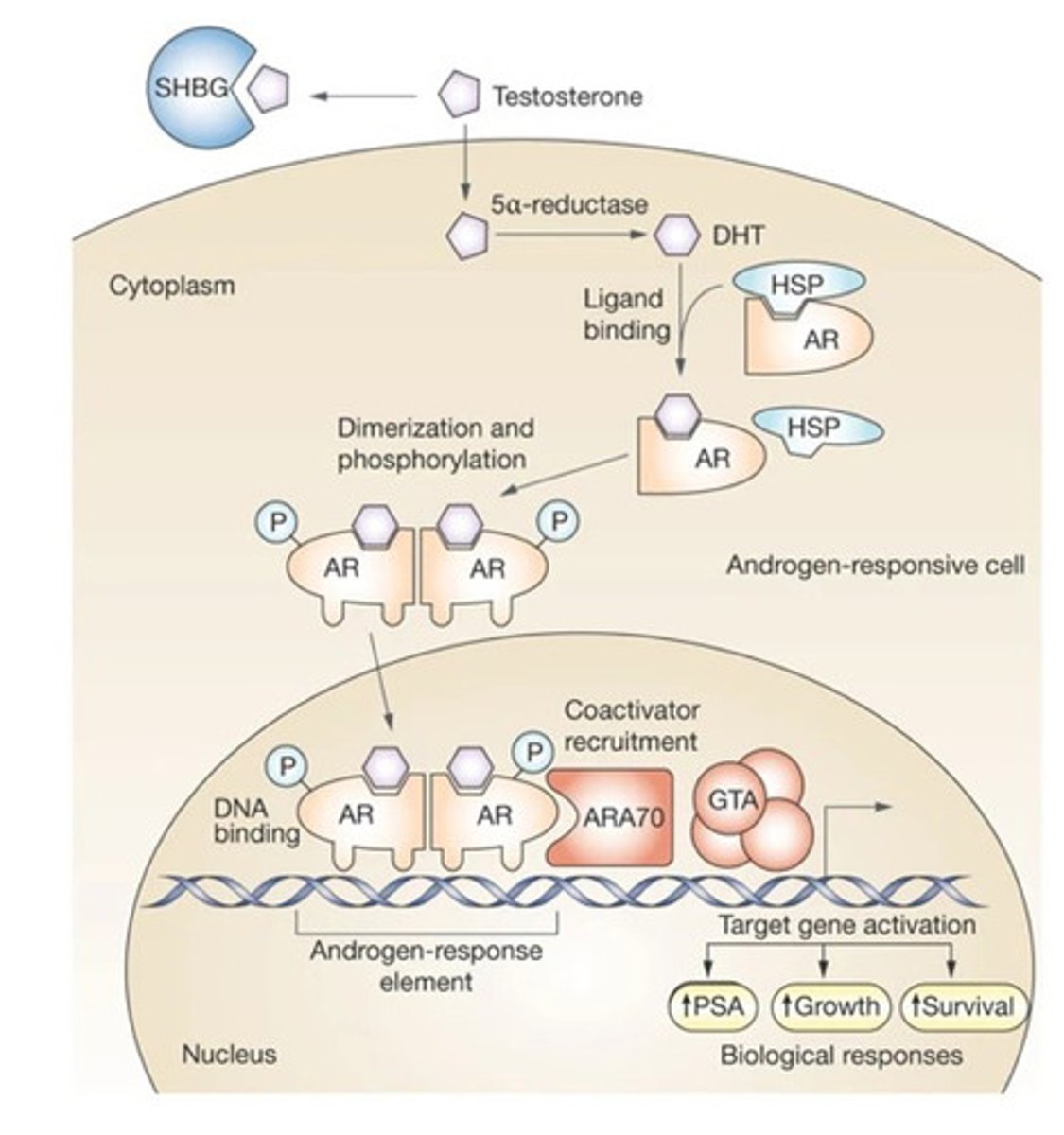
Why does androgen ablation provide therapeutic benefit to prostate cancer?
hint hint: lineage dependence
- Normal prostate epithelium requires a functional androgen receptor (AR) to survive
- Testosterone is taken up by prostate cells and converted to dihydrotestosterone (DHT)
by 5-alpha-reductase
- DHT binds to AR which causes displacement of heat-shock proteins (HSP),
dimerization of AR, nuclear translocation and AR-dependent transcription
- AR target genes promote growth and survival of prostate cells
- AR blockade stops growth of normal and prostate cancer cells
Note: Normal prostate cells are intrinsically dependent on the presence of a functional AR. This dependence is known as lineage addiction (Some tumours are "addicted" to specific lineage genes for survival)and is retained by prostate cancer cells which makes them susceptible to androgen deprivation therapy
other examples of lineage addiction fyi
hematopoietic cells are dependent of the presence of a transcription factor called MYB. However, MYB inhibitors are likely not a viable therapeutic approach for blood cancers since normal blood would also be targeted by such treatments
What are the two strategies to target androgen receptor function?
1) Chemical castration: drug-mediated reduction of circulation androgens—generally
achieved through gonadal and adrenal testosterone synthesis blockade using drugs like
How:
How testosterone is usually produced:
-Most androgens come from testes and some from adrenal glands
-Luteinising hormone-releasing hormone (LHRH/GnRH) stimulates the pituitary gland to release Luteinizing Hormone (LH).
-LH travels to the Leydig cells in the testes and stimulates testosterone production
MOA of LHRH agonists (that carry out chemical castration):
-LHRH agonists initially overstimulate LHRH receptors, leading to a temporary surge ("flare") in LH and testosterone levels = receptor desensitisation (when this is prolonged) = drop in LH and testosterone production = supresses androgen signalling.
Leuprolide/Abiraterone
2) Androgen antagonists: molecules that compete with androgens for binding to AR but
do not activate it—drugs like Bicalutamide/Flutamide/Enzalutamide
What is the main adrenal androgen and what inhibitor (give an example class and example of an inhibitor in that class) can be given to block it?
The main adrenal androgen is DHEA.
CYP17 inhibitors (e.g., Abiraterone) block DHEA synthesis, further reducing androgen levels
What are the 3 strategies to target hormonal oestrogen receptor function to treat breast cancer?
- 1) Aromatase Inhibitors (Ais): Letrozole and Anastrozole decrease oestrogen
production by inhibiting aromatase, the enzyme converting androstenedione to estradiol
- 2) Selective Oestrogen Receptor Degraders (SERDs): Fulvestrant competitively
inhibits oestradiol binding and degrades ER
- 3) Selective Oestrogen Modulator: Tamoxifen competitively binds ER thus
displacing oestrogen and inhibiting ER function
NOTE:
- Breast lineage addiction to ER ((o)estrogen receptor) function underlies all responses to E-R targeted therapies
What 2 methods are used in clincal trials to develop cancer therapeutics?
Clinical trials:
-Trial phases
-Biomarkers
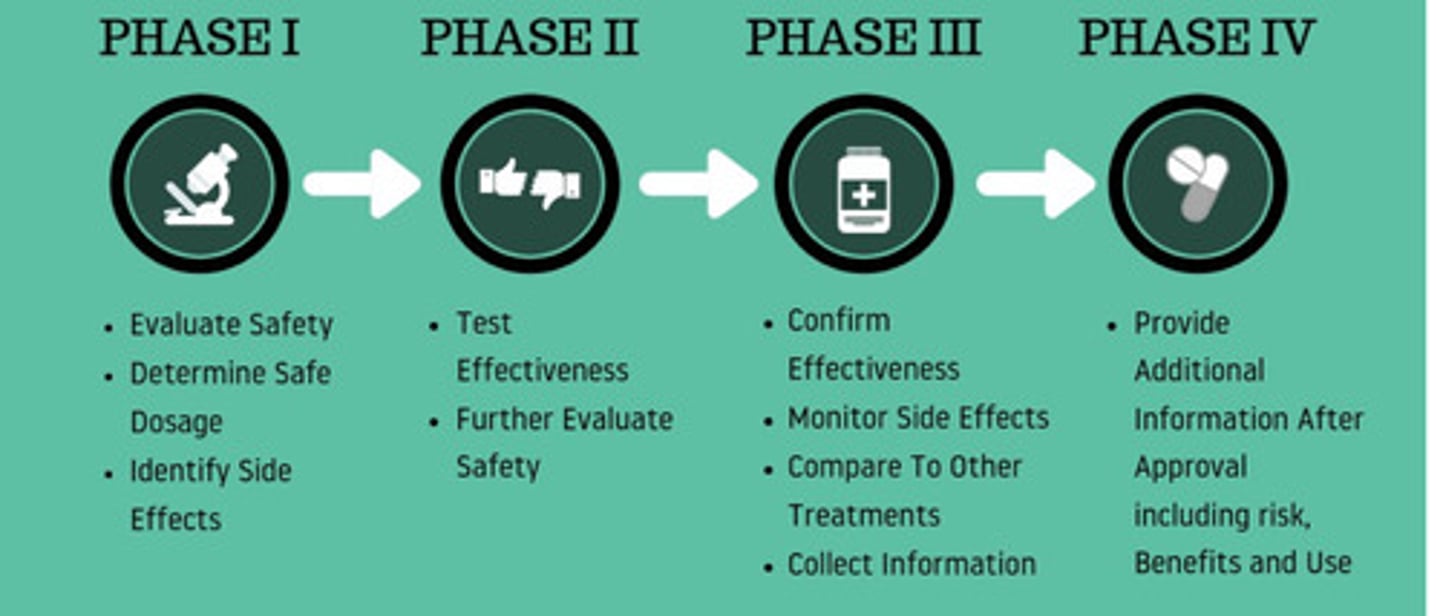
Phases of clinical trial
Phase I: Healthy volunteers
Evaluate safety and side effects
Phase II: Larger group of people
Further evaluate safety and effectiveness
Phase III: Large groups in clinics and hospitals
Effectiveness, side effects, comparisons
Phase IV: Study after drug is marketed
Effectiveness in various populations
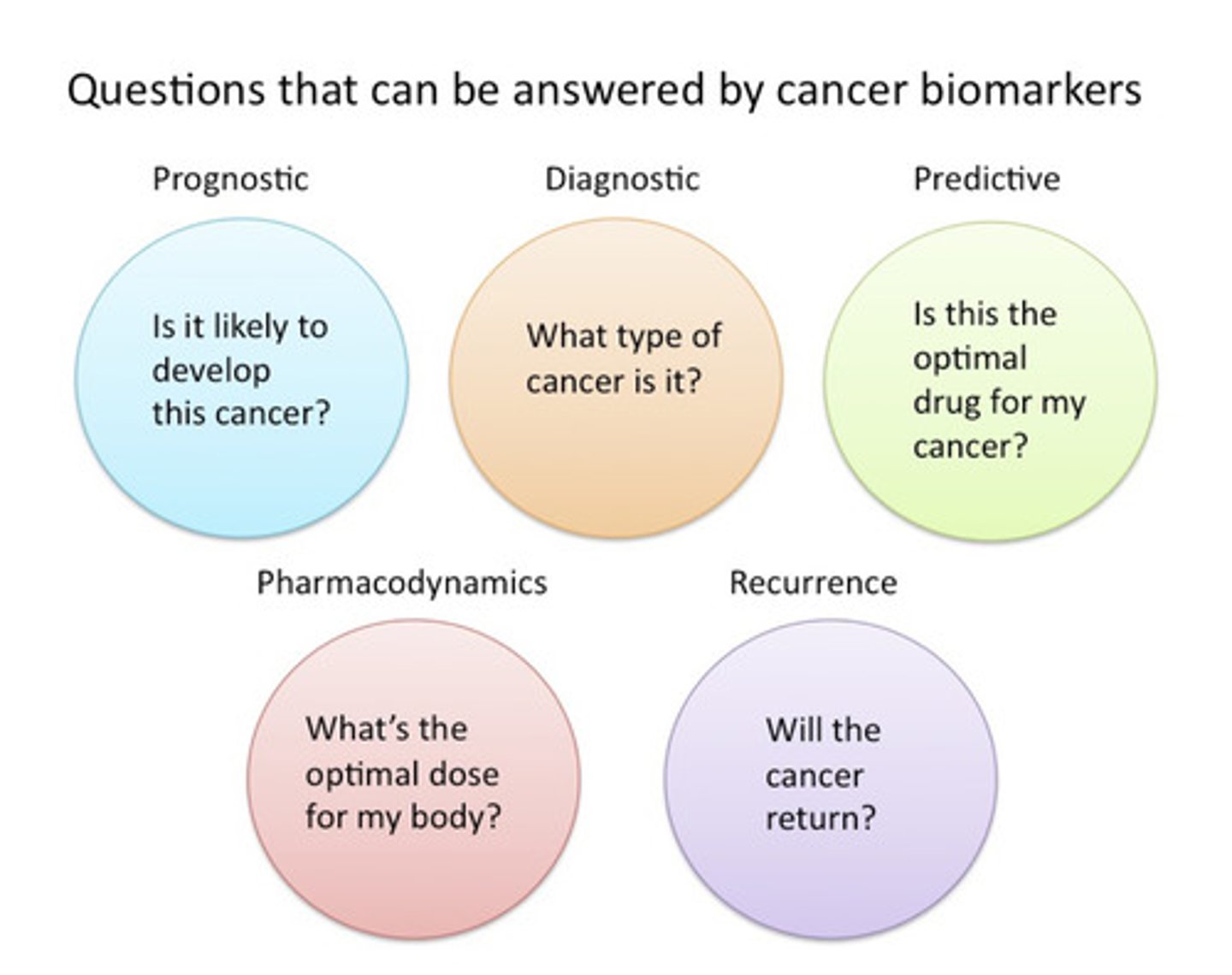
What information can we gather from cancer biomarkers?
Examples:
• Prognostic: Mutations in BRCA1/2, p53, and APC genes all predict high likelihood of developing cancer.
• Diagnostic: Mutations the IDH1 gene can help to classify brain tumour subtypes. Similarly, gene expression signatures captured with the Oncotype kit can help classify types of breast cancer.
• Predictive: Mutations in BRCA1/2, BRAF, EGFR, or KRAS can all help determine whether a patient would benefit from PARP, BRAF, EGFR, or KRAS inhibitors, respectively
Diagnostic = Detects disease.
Prognostic = Predicts disease outcome (regardless of treatment).
Predictive = Predicts treatment response