Preparing Amines
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
two ways we can prepare aliphatic primary amines:
From halogenoalkanes, using a nucleophilic substitution reaction
From nitriles, using a reduction reaction
Category of reaction: :Preparing Amines From Halogenoalkanes
Nucleophilic substitution
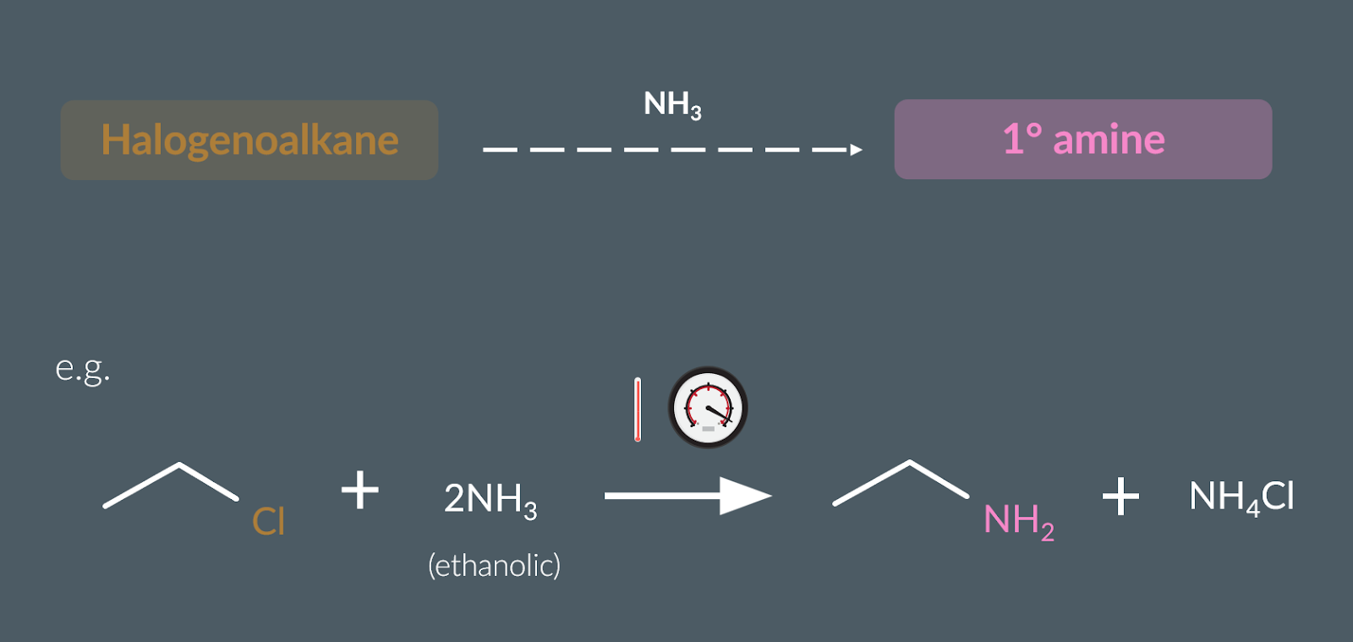
Products: Preparing Amines From Halogenoalkanes
an amine,
a quaternary ammonium salt.
Conditions:Preparing Amines From Halogenoalkanes
heat
high pressure
excess ammonia dissolved in ethanol
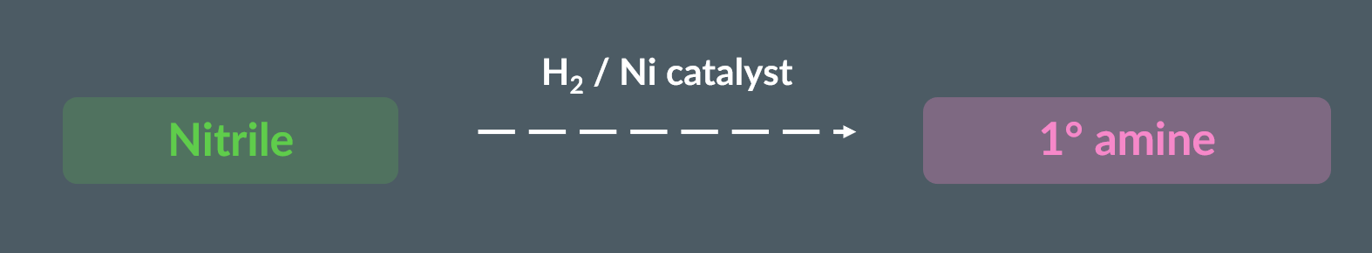
wht to use prepare a primary amine from a nitrile,
use hydrogen and a metal catalyst, such as nickel.
why is Preparing Amines from Nitriles more efficient than the halogenoalkane method
the atom economy is 100%, and because there is no risk of further reactions happening between the primary amine and a halogenoalkane.
also reduce nitriles to amines using
LiAlH4 in dry ether.
CH3CN+4[H]→CH3CH2NH2
prepare aromatic amines
reducing aromatic nitro compounds.
what is used and how to prepare aromatic amines
the reducing agent is a combination of concentrated hydrochloric acid and tin.

equation for this reaction for Preparing Aromatic Amines
C6H5NO2+6[H]→C6H5NH2+2H2O
Aromatic amines used for
to manufacture colourful dyes